Abstract
Objective
To evaluate the effects of change in blood pressure on plasma volume under increased permeability.
Design
Prospective randomized laboratory study.
Subject
Sixty-one adult male Sprague–Dawley rats.
Interventions
Permeability was increased via an anaphylactic reaction by injection of 0.5 ml dextran 70. One hour later, volume expansion with 15 ml/kg of 5% albumin was given for 15 min. Plasma volume was measured just before and 2.5 h after the albumin infusion (125I-albumin tracer technique). The study included a control group, a noradrenalin group and a metoprolol/clonidine group (n = 10 in each group). The vasoactive treatment started after the albumin infusion and continued throughout the experiment. We also investigated the effect of noradrenalin on plasma volume under hypovolemia. Central venous pressure was measured to estimate the venous pressure effect of noradrenalin (n = 6). The results were compared with corresponding plasma volume effects of noradrenalin under normal permeability.
Results
The remaining increase in plasma volume 2.5 h after the albumin infusion was 11.8 ± 3.6 ml/kg in the control group, 0.5 ± 6.3 ml/kg in the noradrenalin group (p < 0.01) and 12.6 ± 4.9 ml/kg in the metoprolol/clonidine group (ns). The loss of plasma volume by noradrenalin under hypovolemia was 3.5 ± 3.0 ml/kg. The remaining increase in plasma volume after the albumin and noradrenalin treatment under normal permeability was 13.7 ± 3.4 ml/kg.
Conclusion
Increase in blood pressure by noradrenalin induces loss of plasma volume, which is much greater under increased than under normal permeability and less pronounced in hypovolemia. According to the two-pore theory of transvascular fluid exchange, the loss may be explained by increased hydrostatic capillary pressure.
Similar content being viewed by others
Introduction
Patients suffering from general inflammation, e.g. after trauma and extensive surgery, during severe infections or following anaphylactic reactions, often develop increased microvascular permeability [1–5]. This may result in transcapillary leakage and hypovolemia, in turn leading to reduced venous return and cardiac output, and generalized vasoconstriction due to activation of the baroreceptor reflex [6]. One goal in the treatment of these patients, therefore, is to maintain an adequate circulating blood volume [5, 7].
The two-pore theory of transcapillary fluid exchange [8] postulates that the loss of plasma fluid and proteins will rise with an increase in hydrostatic capillary pressure, especially when microvascular permeability is increased. Fluid and small solutes pass the capillary membrane through all pores along the entire microvascular bed, whereas proteins pass the membrane through the 10–30 × 103 times less common larger pores of the venous side of the capillary network and in the venules [8]. Due to the low oncotic pressure gradient across the large pores, the transcapillary/transvenular hydrostatic pressure is the major force responsible for fluid flow through these pores, and proteins are lost mainly via convection when following the large-pore fluid stream. The protein loss is dependent on both the number and size of large pores (large pore permeability) and the hydrostatic capillary pressure. This theory means that the loss of plasma fluid and proteins will increase with an increase in arterial pressure due to the simultaneous increase in the hydrostatic capillary pressure, especially at a state of inflammation when permeability is increased and autoregulation of hydrostatic capillary pressure is depressed [9]. The hypothesis offers a potential therapeutic strategy to reduce the need for blood volume substitution in critically ill patients by avoiding high arterial pressures.
The aim of the present study was to evaluate the extent to which arterial pressure influences the plasma volume loss during an inflammatory state with increased permeability, comparing the results with those obtained at a non-inflammatory state with normal permeability. Arterial pressure was increased by noradrenalin infusion, and a mixture of the anti-hypertensive substances metoprolol and clonidine was given to reduce arterial pressure. Permeability was increased by injection of a small dose of dextran, which is known to cause an anaphylactic reaction with plasma leakage due to increased permeability in the rat [10, 11].
Methods
Materials and anesthesia
The study was approved by the local ethics committee for animal research, and the animals were treated in accordance with the Guidelines of the National Institutes of Health for Care and Use of Laboratory Animals. Adult male Sprague–Dawley rats (n = 61), weighing 349 ± 11 g were used. The animals were tracheostomized under isoflurane anesthesia (Forene; Abbot, Stockholm) via a facemask and connected to a ventilator (Ugo Basile; Biological Research Apparatus, Comerio, Italy) using a PEEP of 2.5 cm H2O. Anesthesia was maintained by isoflurane (1.5–1.8%) through the tracheal cannula. Body core temperature measured rectally was maintained at 37.1–37.2 °C via a feedback-controlled heating pad. End-tidal PCO2 was monitored continuously and kept between 4.7 and 5.2 kPa (Capstar-1000; CWE, Ardmore, PA). The left femoral artery was cannulated for measurement of arterial blood pressure and for blood sampling for measurement of arterial blood gases, hematocrit and electrolytes (i-STAT; Hewlett-Packard, Böblingen, Germany). The left femoral vein was cannulated and used for injections and infusions. The animals were killed by decapitation.
Experimental protocol
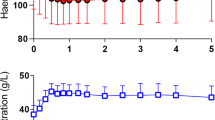
The experimental protocol is shown in Fig. 1. The main study included three groups with increased permeability with 10 rats in each, where the plasma volume-expanding effect of 5% albumin was studied at three different arterial pressure levels during a state of increased permeability. Increased permeability was achieved by an intravenous injection of 0.5 ml dextran 70 (Macrodex 6%; Pharmalink AB, Upplands Väsby, Sweden) given after the surgical preparation [10]. The albumin solution at a volume of 15 ml/kg was given over 15 min at 1 h after the dextran injection, a time point at which the decrease in plasma volume had reached its maximum and arterial pressure was significantly reduced (Fig. 2) [11]. In group 1, no vasoactive drugs were given (control group). In group 2, noradrenalin infusion was started after the albumin infusion with the purpose of increasing arterial pressure to a clinically relevant level just above baseline, and continued throughout the experiment. In group 3, a continuous infusion of a mixture of the beta-1-blocking agent metoprolol (Seloken; Astra Zeneca, Mölndal, Sweden) and the alpha-2 agonist clonidine (Catapressan; Boehringer Ingelheim, Stockholm, Sweden) was started after albumin infusion with the purpose of decreasing arterial pressure. The dose of noradrenalin was in the range of 0.5–2.2 μg/kg/min. After a bolus dose of 1 mg/kg and 1.0 μg/kg of metoprolol and clonidine, respectively, these drugs were infused at a rate of 1.0 mg/kg/h and 1.0 μg/kg/h, doses shown to have effective blood pressure reducing effects under normal circumstances in the rat [12, 13]. The experiments were randomized but not blinded. In a separate group (n = 9), an attempt was made to evaluate the effect of increased arterial pressure by noradrenalin infusion on plasma volume under conditions of hypovolemia and increased permeability. The experiments were similar to those in group 2 above, except that no albumin infusion was given. Central venous pressure was measured via the right internal jugular vein in 12 rats, 6 of which given dextran and albumin as in group 1, and 6 given also noradrenalin as in group 2, to evaluate whether noradrenalin induces an increase in venous pressure, which may have influenced the results. To evaluate the importance of the increased permeability for the plasma volume loss during noradrenalin infusion, the plasma volume loss was investigated also under normal permeability under noradrenalin infusion in a separate group of experiments (n = 10). In these experiments, the rats were bled by 15 ml/kg and restituted with albumin of 15 ml/kg before the initiation of noradrenalin in an attempt to match the situation in group 2, where normovolemia was obtained by albumin infusion from a hypovolemic state.
Mean arterial pressure for the control group (▲), the noradrenalin group (■), the metoprolol/clonidine group (●) and the group given noradrenalin but not albumin (⊠), all with increased permeability, and for the group given albumin and noradrenalin with normal permeability (□). Note that no dextran was given to the latter group and the initial blood pressure fall from baseline was a consequence of bleeding (15 ml/kg). Data presented as mean ± SD. #Difference compared with baseline and after albumin infusion in all groups (p < 0.01). **Difference between noradrenalin groups and control and metoprolol/clonidine groups at same time points (p < 0.01)
Arterial blood gases were measured at baseline, 1 h after the dextran injection before the infusion of albumin, soon after the albumin infusion, and at the end of the experiment (Fig. 1). Plasma volume was measured 1 h after the dextran injection before the infusion of albumin, and at the end of the experiment (Fig. 1).
The plasma volume was calculated by measurement of the increase in radioactivity per milliliter of plasma after an intravenous injection of a known amount of activity of 125I-albumin [14]. Radioactivity was measured with a gamma counter (Wizard 1480; LKB-Wallace, Turku, Finland). The increase in radioactivity was determined by subtracting the activity in a blood sample taken before the injection (from previous plasma volume measurements) from that taken 5 min after the injection. A previous study in septic patients [15] and a study on cats suffering surgical trauma [16] have shown that 5 min recirculation time is enough for complete mixing of the tracer, and the time necessary must be still shorter for the rat with its higher relative circulation rate. After centrifugation, the radioactivity in a fixed volume of plasma was determined. To determine the exact dose injected, the remaining radioactivity in the emptied vial, the syringe and the needles was subtracted from the total radioactivity in the prepared dose. Plasma volume was corrected for plasma volume of the blood samples.
Statistics
Results are presented as mean values ± SD. Statistical comparisons between groups were performed with the non-parametric Mann–Whitney rank sum test and analysis of variance, which, when necessary, was adjusted for multiple comparisons (Bonferroni). P values less than 0.05 were considered significant. Sigma Stat 2.0 software was used.
Results
Physiological data
The data for hematocrit (Hct), sodium (Na+), potassium (K+), pH, PaO2 and PaCO2 are summarized in Table 1. Hct increased from 38.1 ± 1.2% before the dextran infusion to 43.1 ± 2.5% at 1 h after the dextran infusion (p < 0.05) for the whole population in the three main groups (n = 30). After a decrease in Hct by the albumin infusion, the Hct value returned to baseline at the end of the experiment in the noradrenalin group, while it remained low in the control and in the metoprolol/clonidine groups. The Hct value decreased after bleeding/albumin infusion in the group with normal permeability given noradrenalin and had not changed at the end of the experiment. Na+, K+, pH, and PaCO2, were not significantly different between groups. PaO2 was lower in the noradrenalin group both with increased and with normal permeability than in the other groups at the end of the experiment (p < 0.05). Mean arterial blood pressure values at baseline, 1 h after the dextran injection, directly after the albumin infusion, 1 h after the albumin infusion and at the end of the experiment are presented in Fig. 2. Arterial pressure was higher in the noradrenalin than in the control and metoprolol/clonidine groups (p < 0.01). Blood pressures in the control and metoprolol/clonidine groups were lower than baseline values 1 h after the albumin infusion and at the end of the experiment (p < 0.05). The reduction in arterial pressure by metoprolol/clonidine relative to the control group was not statistically significant. In the noradrenalin group not given albumin the increase in blood pressure was, on average, 7–10 mmHg lower than in the noradrenalin group given albumin (Fig. 2). Arterial pressure in the group given noradrenalin with normal permeability was of the same magnitude or slightly above that with increased permeability (Fig. 2). Central venous pressure in experiments with increased permeability in animals given albumin and noradrenalin was 2.6 ± 0.5 mmHg at baseline, 2.4 ± 0.5 mmHg 1 h after the dextran injection, 3.5 ± 0.4 mmHg 20 min after the albumin infusion and 3.5 ± 1.0 mmHg at the end of the experiment (n = 6). These values were not different from those in animals given only albumin, where central venous pressure was 2.7 ± 0.2 mmHg at baseline, 2.3 ± 0.6 mmHg 1 h after the dextran injection, 3.3 ± 0.8 mmHg 20 min after the albumin infusion and 3.2 ± 0.8 mmHg at the end of the experiment (n = 6).
Plasma volume
Normal plasma volume in the male Sprague–Dawley adult rat, as measured in previous studies with the same technique as used in the present study, was 40–42 ml/kg [11, 17]. Plasma volume in the three main groups directly before infusion of albumin was 32.1 ± 3.3 ml/kg (n = 30), with no difference between the groups. The remaining increase in plasma volume 2.5 h after the 15 ml/kg infusion of albumin (PV2–PV1; Fig. 1) is shown in Fig. 3. It was 11.8 ± 3.6 ml/kg in the control group, 0.5 ± 6.3 ml/kg in the noradrenalin group (p < 0.01) and 12.6 ± 4.9 ml/kg in the metoprolol/clonidine group (ns). In the experiments in which noradrenalin was given without plasma volume substitution, the plasma volume had decreased by 3.5 ± 3.0 ml/kg at the end of the experiment. Urine output from start to the end of the experiment ranged from 1.5 ml/kg to 3.5 ml/kg (average 2.7 ml/kg) in the control and the metoprolol/clonidine groups (groups 1 and 3) and from 3.5 ml/kg to 5.5 ml/kg (average 4.8 ml/kg) in the noradrenalin group (group 2).
Increase in plasma volume 2.5 h after the albumin infusion compared to the plasma volume just before the albumin infusion of 15 ml/kg for the three groups given albumin with increased permeability and for the group given noradrenalin and albumin under normal permeability. It can be seen that the noradrenalin group with increased permeability had a much lower increase in plasma volume than the other three groups, which were not significantly different from each other. (n = 10 in all groups). Data presented as mean ± SD. ** p < 0.01
The remaining increase in plasma volume 2.5 h after the infusion of albumin and treatment with noradrenalin in the experiments with normal permeability was 13.7 ± 3.4 ml/kg (Fig. 3).
Discussion
The present study has shown that noradrenalin infusion at a normovolemic state, giving a significant increase in arterial pressure, induces a loss of plasma fluid in the rat, and this loss is much larger when permeability is increased than when permeability is normal. The blood pressure reduction by metoprolol/clonidine did not become statistically significant with the doses used and had no significant effect on plasma volume. The results are compatible with the observed changes in Hct, with an increase in Hct in the noradrenalin group and no significant change in Hct in the control and metoprolol/clonidine groups. The plasma volume-reducing effect by noradrenalin was less pronounced under hypovolemia than under normovolemia. Arterial oxygenation was impaired in the two noradrenalin groups (both under increased and normal permeability) compared with the other groups.
This model of dextran-induced increased permeability has limitations in the sense that it does not represent the whole complexity of septic inflammation. This fact, however, is of advantage in the present study as the purpose was to evaluate the effects of an isolated increase in permeability.
The tracer albumin technique used is well established for measurement of plasma volume [11, 15, 16, 18, 19], and has been used previously under normal as well as inflammatory states on the rat, the cat and man [11, 15, 16, 19]. The potential errors in the technique, such as effects of poor mixing of the tracer in plasma and effects of the transcapillary escape during the mixing period (5 min), have been discussed previously and found to be small [11].
As mentioned in the Introduction, a larger transcapillary leakage of plasma fluid under increased permeability than under normal permeability, reflected as plasma volume loss of about 14 ml/kg at increased permeability and about 1.5 ml/kg at normal permeability, is compatible with the two-pore theory of transcapillary fluid exchange [8]. Even a minute increase in total pore area from normal by way of an increase in large pore area may cause a marked increase in leakage of proteins from the continuous small leakage at normal permeability (the latter reflecting the normal transcapillary escape rate) [8]. The fast dextran-induced plasma volume loss may exemplify such an increase in leakage. The permeability-increasing effect of dextran has only been demonstrated indirectly, however, by the visually observed marked edema in the legs and around the neck developing shortly after the dextran injection, and the large reduction in plasma volume in combination with increased Hct [10, 11].
The infusion of noradrenalin resulted in an increase in blood pressure from subnormal to values slightly above those at baseline (Fig. 2), an increase which is relevant also from a clinical point of view. The simultaneous increase in plasma volume loss cannot be explained by a direct permeability-increasing effect of noradrenalin, as corresponding plasma volume loss was much smaller at normal permeability, and no permeability-increasing effects have been shown by α-stimulation and, if anything, permeability is decreased by β-stimulation [20]. The higher urine volume by 2–2.5 ml/kg in the noradrenalin group than in the control group, of which 20–25% comes from the intravascular space, means that only 0.5–1 ml/kg of the higher plasma volume loss by noradrenalin can be explained by the increase in urine production.
The fact that there was no further preservation in plasma volume in the metoprolol/clonidine group compared with the control group was an expected finding, as arterial pressure was not reduced (Fig. 2). Lack of a significant reduction in arterial pressure by metoprolol/clonidine most likely can be explained by the specific dextran-induced hypovolemic situation with a low arterial pressure from the outset with difficulties of further reduction in arterial pressure, but we cannot exclude that the reduction had been greater with higher doses of metoprolol/clonidine. The plasma volume loss in the metoprolol/clonidine group, however, was already so small (about 2.5 ml/kg) that only minor further reduction had been obtained.
As an increase in venous pressure will be transferred to the capillaries (by about 80%) [21], noradrenalin infusion may also influence hydrostatic capillary pressure through alterations of venous pressure, e.g. due to its vasoconstrictor effects on the venous capacitance vessels. Such an increase in hydrostatic capillary pressure may have contributed to the noradrenalin-induced plasma volume loss, an effect also compatible with the two-pore theory. In the present study, however, such a venous effect must be small, as the average central venous pressure was only slightly higher in the noradrenalin than in the control group (see Results).
The loss of plasma volume when noradrenalin was given without prior albumin infusion (uncorrected hypovolemia) at increased permeability was only 3–4 ml/kg, against a loss of 14 ml/kg in group 2 when noradrenalin was given after resuscitation with albumin (by 15 ml/kg). The loss was only moderately larger than the loss of plasma volume when neither noradrenalin nor albumin was given, which was virtually zero, as shown in a previous study [11]. In other words, the higher plasma volume, the greater will be the plasma volume loss by an increase in arterial pressure, at least at a state of increased permeability. We can only speculate about the underlying mechanisms. The difference in urine production of 2–4 ml/kg between the normovolemic and the hypovolemic groups can explain up to 1 ml/kg of the difference in plasma volume loss. There may also be a difference in the increase in hydrostatic capillary pressure caused by noradrenalin when given from a normovolemic than from a hypovolemic state. For example, the somewhat lower arterial pressure (Fig. 2) and a smaller venous pressure in the group not given albumin and a possible difference in post-/precapillary resistance ratio may reduce the plasma volume loss. There may also be a plasma volume-preserving effect by the higher Hct value in the group not given albumin [18, 19].
In addition to arterial and venous pressures, the hydrostatic capillary pressure is also a function of the post-/precapillary resistance ratio [21]. It is well known that noradrenalin induces vasoconstriction not only in small arteries and arterioles but also in venules [21]. This means that the decrease in post-/precapillary resistance ratio may be small in spite of the fact that there was a large increase in total vascular resistance. Thus, the noradrenalin-induced increase in arterial pressure most likely results in a net increase in hydrostatic capillary pressure in spite of its precapillary vasoconstrictor effect.
The mechanisms underlying the worse arterial oxygenation in the two noradrenalin groups compared with the control and metoprolol/clonidine groups (Table 1) are not clear. It may be an effect of increased lung water and/or uneven lung perfusion in combination with α-mediated vasoconstriction.
In conclusion, the present study on rat has shown that an increase in arterial pressure achieved by noradrenalin infusion increases the loss of plasma volume during a state of increased permeability. The corresponding loss was much smaller during a state of normal permeability. The plasma volume loss was smaller in uncorrected hypovolemia. Providing the results can be transferred to man, they suggest that avoidance of high blood pressures may save plasma volume and reduce the need for plasma expanders in patients with increased permeability. The results are compatible with the so-called two-pore theory for transvascular fluid exchange.
References
Brun-Buisson C (2000) The epidemiology of the systemic inflammatory response. Intensive Care Med 26:S64–S74
Fishel RS, Are C, Barbul A (2003) Vessel injury and capillary leak. Crit Care Med (Suppl) 31:S502–S501
Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, Calman KC (1985) Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet 1:781–784
Groeneveld AB, Teule GJ, Bronsveld W, van den Bos GC, Thijs LG (1987) Increased systemic microvascular albumin flux in septic shock. Intensive Care Med 13:140–142
Imm A, Carlson RW (1993) Fluid resuscitation in circulatory shock. Crit Care Clin 9:313–333
Guyton A, Hall J (2000) Textbook of medical physiology. Saunders, Philadelphia
Kreimeier U (2000) Pathophysiology of fluid imbalance. Crit Care (Suppl 2) 4:S3–S7
Rippe B, Haraldsson B (1994) Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev 74:163–219
Mellander S, Maspers M, Björnberg J, Andersson LO (1987) Autoregulation of capillary pressure and filtration in cat skeletal muscle in states of normal and reduced vascular tone. Acta Physiol Scand 129:337–351
Guo Y, Hedquist P, Gustafsson LE (2001) Absence of mast cell involvement in active systemic anaphylaxis in rats. Eur J Pharmacol 430:305–310
Dubniks M, Persson J, Grände P-O (2007) Plasma volume expansion of 5% albumin, 4% gelatin, 6% HES 130/0.4 and normal saline under increased microvascular permeability in the rat. Intensive Care Med 33:293–299
Wendt RL, Much DR, Bergey JL, Wicks TC (1981) Comparative antihypertensive effects of propranolol and metoprolol in DOC-treated rats. Arch Int Pharmacodyn Ther 253:110–120
Ally A (1998) Cardiovascular effects of intravenous administration of clonidine in conscious rats. Brain Res 810:153–160
Valeri CR, Cooper AG, Pivacek LE (1973) Limitations of measuring blood volume with iodinated I-125 serum albumin. Arch Intern Med 132:534–538
Margarson MP, Soni NC (2005) Plasma volume measurement in septic patients using an albumin dilution technique: comparison with the standard radio-labelled albumin method. Intensive Care Med 31:289–295
Persson J, Grände P-O (2006) Plasma volume expansion and transcapillary fluid exchange in skeletal muscle of albumin, dextran, gelatin, hydroxyethyl starch, and saline after trauma in the cat. Crit Care Med 34:2456–2462
Lundin S, Folkow B, Rippe B (1981) Central blood volume in spontaneously hypertensive rats and Wistar-Kyoto normotensive rats. Acta Physiol Scand 112:257–262
Valeri CR, Donahue K, Feingold HM, Cassidy GP, Altschule MD (1986) Increase in plasma volume after the transfusion of washed erythrocytes. Surg Gynecol Obstet 162:30–36
Persson J, Grände P-O (2005) Volume expansion of albumin, gelatin, HES, saline and erythrocytes after haemorrhage in rat. Intensive Care Med 31:296–301
Möller AD, Grände P-O (1999) Role of prostacyclin and nitric oxide in regulation of basal microvascular hydraulic permeability in cat skeletal muscle. J Vasc Res 36:245–252
Mellander S, Johansson B (1968) Control of resistance, exchange, and capacitance function in peripheral circulation. Pharmacol Rev 20:117–196
Acknowledgements
This study was supported by grants from the Swedish Research Council (#11581) and from the Medical Faculty of Lund University, Sweden. We are grateful to Helén Davidsson for her highly skilled technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-007-0757-1.
Rights and permissions
About this article
Cite this article
Dubniks, M., Persson, J. & Grände, PO. Effect of blood pressure on plasma volume loss in the rat under increased permeability . Intensive Care Med 33, 2192–2198 (2007). https://doi.org/10.1007/s00134-007-0756-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0756-2