Abstract
Objective
To characterize empiric antibiotic use in patients with suspected nosocomial ICU-acquired infections (NI), and determine the impact of prolonged therapy in the absence of infection.
Design and setting
Multicenter prospective cohort, with eight medical-surgical ICUs in North America and Europe.
Patients
195 patients with suspected NI.
Methods
The diagnosis of NI was adjudicated by a blinded Clinical Evaluation Committee using retrospective review of clinical, radiological, and culture data.
Results
Empiric antibiotics were prescribed for 143 of 195 (73.3%) patients with suspected NI; only 39 of 195 (20.0%) were adjudicated as being infected. Infection rates were similar in patients who did (26 of 143, 18.2%), or did not (13 of 52, 25.0%) receive empiric therapy ( p = 0.3). Empiric antibiotics were continued for more than 4 days in 56 of 95 (59.0%) patients without adjudicated NI. Factors associated with continued empiric therapy were increased age ( p = 0.02), ongoing SIRS ( p = 0.03), and hospital ( p = 0.004). Patients without NI who received empiric antibiotics for longer than 4 days had increased 28-day mortality (18 of 56, 32.1%), compared with those whose antibiotics were discontinued (3 of 39, 7.7%; OR = 5.7, 95% CI 1.5–20.9, p = 0.005). When the influence of age, admission diagnosis, vasopressor use, and multiple organ dysfunction was controlled by multivariable analysis, prolonged empiric therapy was not independently associated with mortality (OR = 3.8, 95% CI 0.9–15.5, p = 0.07).
Conclusions
Empiric antibiotics were initiated four times more often than NI was confirmed, and frequently continued in the absence of infection. We found no evidence that prolonged use of empiric antibiotics improved outcome for ICU patients, but rather a suggestion that the practice may be harmful.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection is a common cause and complication of critical illness. The highest rates of nosocomial infection (NI) occur in the intensive care unit [1], with an estimated incidence between 10 and 45% of intensive care unit (ICU) admissions [2–4]. Ventilator-associated pneumonia affects 10–15% of ICU patients [5, 6], whereas bloodstream, urinary tract, and surgical site infections occur somewhat less frequently [3, 4, 7]. The ICU-acquired infection generate costs that are three times greater than those of community-acquired infection in the ICU [8]; estimates of attributable mortality range from 0 [5, 9] to 35% [3]. Risk factors include severity of illness, neurological compromise, the use of invasive devices, and prior exposure to antibiotics [10, 11]. The diagnosis of ICU-acquired infection is notoriously difficult. The clinical and laboratory manifestations are non-specific, infection is difficult to differentiate from colonization, and concomitant use of antibiotics may render culture results unreliable.
These factors – a high prevalence of a diagnostically challenging disease whose morbidity and mortality is considerable – combine to make antibiotics one of the most widely used class of drugs in the ICU [12]. The prevalence of antibiotic use in critically ill patients is as high as 60% [4, 13], and rates of prescription in the ICU are estimated to be ten times higher than on general hospital wards [14]. Empiric therapy, defined as the pre-emptive administration of antibiotics prior to microbiological documentation of infection, is second only to surgical prophylaxis as the most common indication for initiating antibiotics in the ICU: as many as 40% of patients receive at least one course of empiric treatment during their ICU stay [15]. The benefits of empiric therapy are inferred from cohort studies that suggest that survival is improved when antibiotics appropriate to the subsequently identified infecting organism are administered as soon as possible after the diagnosis is suspected [16–20].
Indiscriminate antibiotic use, however, is also associated with an increased prevalence of antibiotic-resistant organisms in the ICU, and patients exposed to broad-spectrum agents are at increased risk of developing super-infections with resistant organisms [21]. As a consequence, many authors advocate the use of early broad spectrum empiric therapy, with de-escalation of antibiotics based on the results of cultures and sensitivities [22, 23]. Recent randomized trials have suggested that restricting antibiotic exposure can decrease rates of superinfections and improve survival [24, 25], and limit toxicity and unnecessary costs.
We used a database of critically ill ICU patients with suspected infection who had been recruited to a prospective study of the diagnostic utility of a rapid assay of bacterial endotoxin [26]. Our primary objective was to characterize antibiotic prescribing practices in patients with suspected ICU-acquired infection. Our secondary objective was to determine whether the restriction of empiric antibiotic therapy based on culture results influences outcome [27].
Methods
Patients
The MEDIC study was a multicenter international prospective cohort study of 526 ICU patients with suspected infection (both community and ICU-acquired) conducted between January and August 2000 [26]. Patients were recruited from the medical and surgical ICU's of 8 university-affiliated, tertiary care hospitals (two in Europe, four in Canada, and two in the U.S.). All underwent diagnostic cultures, radiological evaluation, and/or an invasive diagnostic procedure for suspected infection. The protocol was approved by the institutional review boards of each participating institution, and written informed consent was obtained from each patient or a surrogate decision-maker.
Data collection
Baseline demographic and daily clinical data, including APACHE II [28] and Multiple Organ Dysfunction (MOD) [29] scores, radiographic and microbiological data, and antibiotic administration were recorded electronically for all patients. Patients were followed daily for 7 days or until death or discharge from the ICU. The primary outcome was 28-day all-cause mortality.
Diagnosis and adjudication of infection
A clinical evaluation committee (CEC), blinded to outcome status, reviewed data from all patients who had positive cultures, using Centers for Disease Control (CDC) criteria for nosocomial infection (Appendix A in ESM) [30]. The CEC was made up of five critical care physicians from North America and Europe (Appendix B in ESM). Cases were first reviewed by two critical care fellows or attending intensivists. If these two individuals agreed on the presence or absence of infection, the case was reviewed by one additional CEC member and concordance with the initial pair of evaluators resulted in final adjudication of infectious status. If the CEC member disagreed with the initial review, or if the two initial reviewers disagreed, the case was adjudicated by two CEC members. Agreement between this second set of CEC members constituted a final adjudication. Any persistent disagreement resulted in formal review and discussion of the case by all members of the CEC, where a simple majority opinion of the members led to assignation of final infectious status.
Definitions
Nosocomial ICU-acquired infection (NI) was defined as any infection that developed at least 48 h following admission to the ICU. Empiric antibiotic therapy was defined as therapy initiated prior to the availability of microbiological evidence of infection, and within 24 h of the time when diagnostic tests for infection were obtained. We considered antibiotics to be directed therapy when diagnostic test results were available prior to the institution of therapy.
Inadequate empiric therapy was defined as prescription of an empiric regimen with insufficient spectrum to target the class of organism subsequently identified by culture results. Prolonged empiric therapy was defined as empiric antibiotic therapy that was continued for more than 4 days in the absence of adjudicated infection. Uninfected patients who never received empiric therapy, or had their empiric antibiotic therapy discontinued by the fourth study day were considered to have received restrictive therapy.
Statistical analysis
We conducted univariate analyses to identify factors associated with the decision to initiate empiric antibiotics, and the decision to continue empiric therapy in the absence of infection. The chi-squared test was used to compare proportions, and Student's t-test was used for continuous variables. Factors associated with the initiation or continuation of empiric therapy with a p-value less than 0.10 on univariate analysis were then entered into a multivariable binary logistic regression model using backward Wald selection.
To examine the relationship between antibiotic use and patient outcomes, we first evaluated the association between clinical variables and 28-day mortality using chi-squared tests and t-tests as appropriate. We then conducted a multivariable logistic regression model as described above. The binary variable of presence or absence of nosocomial infection was forced into the model to assess the impact of acquiring a nosocomial infection on mortality after controlling for all other potentially confounding variables.
To evaluate the impact of restrictive empiric therapy on mortality, we included all patients without infection in a second multivariable analysis comparing patients who received prolonged therapy with those who had received restrictive therapy. Patients who died or were discharged by day 4 were excluded, because we could not determine whether they met criteria for restrictive therapy. Potential confounding variables were entered into a multivariable binary logistic regression model to identify the independent contribution of ongoing empiric antibiotic therapy on 28-day mortality. We used clinical variables from day 3 in this latter analysis to provide a more accurate reflection of the impact of continuation of therapy beyond day 4. Results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). A p-value of less than 0.05 was considered significant and all tests were two tailed. Statistical analyses were conducted with SPSS statistical software (SPSS, Chicago, Ill.).
Results
Demographic characteristics, prevalence, and outcomes of infection
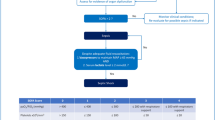
Of the 526 patients, 195 (37.1%) met criteria for suspected NI, and 331 (62.9%) met criteria for suspected community-acquired infection (CAI). The baseline demographic characteristics of the population are presented in Table 1. The 28-day mortality rate for the entire cohort was 27.9%, and was significantly higher for patients with suspected CAI (31.4%, 104 of 331) than for patients with suspected NI (22.1%, 43 of 195); OR = 1.62 (1.07–2.44, p = 0.021). Mortality was also higher for patients adjudicated to have infection (38.1%, 40 of 105) than for those adjudicated not to be infected (25.4%, 107 of 421); OR = 1.80 (1.15–2.83, p = 0.01).
The outcome of the infection adjudication process is shown in Fig. 1. Rates of infection as adjudicated by the CEC were similar for patients with suspected community-acquired infection (CAI; 66 of 331, 19.9%) and suspected NI (39 of 195, 20%). At least one organism was cultured from 103 of 195 (52.8%) patients with suspected NI. The most common sites of adjudicated NI were pneumonia (27 of 39 patients; 69.2%), and primary or secondary bacteremia (12 of 39 patients; 30.8%; Table 2).
Infectious status of all patients with community-acquired or nosocomial infection was adjudicated using a formal process as described in Methods. Data from all patients with positive cultures were reviewed by two clinician reviewers, and if they agreed on infectious status, by one member of the CEC. Concordance of the reviewers and one CEC member was sufficient to establish infectious status (146 of 323 cases, 45.2%). When there was disagreement between the two primary reviewers, data were evaluated by two CEC members; CEC concordance established infectious status
Initiation of empiric antibiotic therapy for suspected nosocomial infection
Empiric antibiotics were prescribed for 143 of 195 (73.3%) of patients with suspected NI (Fig. 2). Clinical factors that on univariate analysis were significantly associated with a decision to initiate empiric antibiotic therapy were an increased white blood cell count (WBC; p = 0.007) and an elevated APACHE II score ( p = 0.028; Table 3). Multivariable analysis showed that the most important independent predictors of administration of empiric therapy were hospital, with significantly less empiric therapy in hospital 2 ( p = 0.01), and WBC ( p = 0.05).
Impact of adequacy of initial empiric antibiotic therapy on outcome from nosocomial infection
The NI was adjudicated to be present in 18.2% (26 of 143) of patients who received empiric antibiotics, and 25.0% (13 of 52) of those who did not ( p = 0.3; OR = 0.6, 0.3–1.4). For the 39 patients meeting adjudicated criteria for infection, only 19 of 39 (48.7%) received adequate empiric coverage, whereas 7 of 39 (17.9%) received inadequate antibiotic therapy for the pathogen identified, and 13 of 39 (33.3%) did not receive any empiric therapy (Fig. 2).
Mortality rates did not differ significantly for patients with NI who received adequate (4 of 19, 21%), as compared with inadequate or no empiric therapy (7 of 20, 35%; p = 0.53), although the numbers were small. Patients who received inadequate or no empiric antibiotics had a modest, but statistically insignificant, increased risk of mortality when compared with patients who received adequate therapy (OR = 2.02, 0.48–8.48, p = 0.33).
Duration of empiric therapy in patients without infection
Empiric antibiotics were initiated for 75.0% of the 156 patients who were adjudicated not to be infected. The percentage of patients remaining on empiric therapy was 68.6% at 72 h, and 59.0% at 5 days. Over the 7-day study with censoring of patients who died or were discharged from the ICU, the proportion of patients remaining on empiric antibiotics for this episode of suspected infection was 41 of 80, or 51.3% (Fig. 3); thus, we found that clinicians often fail to discontinue antibiotic therapy in response to the results of negative diagnostic investigations.
Empiric antibiotics were initially prescribed to roughly comparable numbers of patients who were adjudicated as being infected (solid line) or not infected (dashed line). As culture data became available, rates of antibiotic prescription to infected patients increased, while greater than half of those patients adjudicated as not being infected were still receiving empiric therapy by day 7 (Denominator censored for death and discharge from the ICU)
Factors associated with prolonged empiric antibiotic therapy
On day 5, 95 patients who were adjudicated as not infected remained in the ICU. Empiric therapy had been discontinued in 39 of these patients, but was continued in the remaining 56 patients. Independent predictors of a decision to prolong therapy beyond day 4 included increased age ( p = 0.02), persistence of criteria for the systemic inflammatory response syndrome (SIRS; p = 0.03), and hospital (Table 5). A more restrictive strategy of empiric antibiotic therapy was evident in hospitals 2 ( p = 0.004) and 5 ( p = 0.004), when compared with the other sites.
Impact of empiric antibiotic strategy on outcome
Factors associated with mortality on univariate analysis are shown in Table 5. An adjudicated diagnosis of NI was not significantly associated with mortality ( p = 0.30). On multivariable analysis independent predictors of mortality included increasing age (OR = 1.04, 1.01–1.061, p = 0.002) with an increased odds of death of 1.04 for each additional 1 year of age, MODS score on day 1 (OR = 1.17. 1.07–1.37, p = 0.02) with an increased odds of death of 1.17 for each additional point in MODS score, medical admission type (OR = 3.50, 1.58–6.74, p = 0.003) when compared to surgical admission, and vasopressor use on day 1 (OR = 2.64, 1.11–6.27, p = 0.03. Nosocomial infection was not significantly associated with mortality when forced into this model (OR = 0.95, 0.37–2.47, p = 0.9).
For patients adjudicated as not having NI, the 28-day mortality rate was higher for those patients who received prolonged empiric antibiotic therapy (18 of 56, 32.1%) than for those in whom antibiotics were discontinued within the first 4 days of the study (3 of 39, 7.7%, OR = 5.68, 1.54–20.95, p = 0.005). When potential confounders, including MOD score at day 3, age, medical admission, and vasopressor use on day 3 were controlled for in a multivariable model, the association between prolonged therapy and mortality was no longer statistically significant (OR = 3.75, 0.91–15.49, p = 0.07).
Discussion
It is common practice to administer broad-spectrum empiric antibiotics to critically ill patients who are suspected on clinical grounds of harboring nosocomial infection. Of the cohort of patients reported here, fully 73% received empiric therapy. The factors associated with a decision to start empiric therapy included leukocytosis and an increased APACHE II score; however, the most important determinant of the decision to start empiric treatment was the treating hospital, suggesting that there is considerable variability in the clinical threshold for initiating therapy.
The true prevalence of nosocomial infection is difficult to estimate, because of variability in diagnostic criteria and the confounding effects of previously administered antibiotics on culture results. We adjudicated episodes of NI using an expert clinical evaluation committee who undertook a rigorous review of clinical, microbiological, and radiological data. Using this approach, a diagnosis of invasive infection was supported in only 20% of patients; thus, it would appear that empiric therapy is initiated much more frequently than invasive infection is diagnosed. In a multicenter study of 481 patients in New Zealand and Australia only 46 (25.1%) of 183 patients who received antibiotics empirically for a suspicion of infection subsequently proved to have culture-documented infection [15].
We further found that empiric antibiotic therapy is often continued once culture results become available, even though cultures are negative. Failure to discontinue empiric therapy may be a consequence of the perception that clinical improvement implies a favorable therapeutic response, whereas deterioration suggests a persistent or occult infection [31, 32]. In an observational study of broad-spectrum empiric antibiotic therapy for suspected sepsis, followed by de-escalation of antibiotics based on eventual microbiological results, it was reported that 123 of 157 patients who received empiric imipenem and gentamicin had negative culture results; however, antibiotics were discontinued in only 37% of patients [33]. Even when invasive diagnostic procedures, such as bronchoscopy, are used to diagnose pneumonia, when culture results are negative or inconclusive, antibiotics that were initiated empirically are rarely discontinued [34, 35, 36]. In our study, clinical factors associated with a decision to continue empiric antibiotics included increased illness severity reflected in an increased APACHE II score at baseline and MOD score on day 3, increased age, persistent clinical signs of inflammation or SIRS, and hospital site – variables reflecting both severity of illness and interinstitutional variability in management strategies. At the time of this study no hospital site was using a specific protocol for the discontinuation of empiric therapy, but some variation may have been accounted for by the difference in case mix: the two hospitals most apt to discontinue empiric therapy had a higher proportion of trauma and cardiac cases. Perhaps surprisingly, clinical evidence of septic shock reflected in hypotension and vasopressor use was not associated with this decision.
Finally, this study raises the possibility that prolonging empiric antibiotic therapy in the absence of objective evidence of infection may lead to a worse outcome. This observation is consistent with findings of a randomized controlled trial of patients with suspected ventilator-associated pneumonia randomized to restrictive empiric therapy (ciprofloxacin with discontinuation if cultures were negative by 48 h) or standard therapy dictated by the treating physician (typically broad-spectrum therapy, continued for 10–14 days without consideration of culture results) [25]. Patients randomized to the restrictive strategy had significantly fewer superinfections and infections with resistant organisms, and a statistically insignificant improvement in survival. Alternatively, the findings of our study may suggest that patients who have ongoing inflammatory signs without evidence of a treatable pathogen may have a worse prognosis regardless of antibiotic administration.
Although our study is underpowered to permit strong conclusions regarding the impact of empiric antibiotic therapy on outcome, it illustrates that empiric antibiotic therapy is frequently inadequate, and that antibiotics are commonly continued indiscriminately in ICU patients with suspected infection. Although much attention has focused on the risks of under-use of antibiotics for patients with culture proven infection [16–20], culture results are difficult to predict at the bedside [33, 37, 38], and as this study illustrates, are more likely to be negative or non-contributory in the critically ill patient with suspected nosocomial infection. The occurrence of culture documented nosocomial infection is relatively rare compared with the frequency of empiric antibiotic prescription.
Cohort studies such as this one suffer from inherent weaknesses that preclude valid assumptions of causality. Differing hospitals may treat significantly different patient populations, and face local differences in the rate and microbiology of nosocomial infection. Patients are only examined at one time point and all potentially known and unknown confounding variables cannot be controlled for. For example, antibiotic use before and after the study period was not recorded, and was not controlled for in the analysis. Although it may be assumed that there is consistency in the manner antibiotics are prescribed over an individual patient's stay in the ICU, numerous ICU physicians with potentially different prescribing practices may be involved in the care of a given patient [39]. This factor has not been measured, controlled for, or discussed in previous observational studies evaluating antibiotic use in the ICU. Our process of adjudicating episodes of infection was rigorous, but such rigor may have excluded patients who were truly infected but failed to meet our diagnostic criteria (for example, because of the confounding effects of antibiotics at time of culturing or missing data).
Conclusion
In conclusion, we show that nosocomial ICU-acquired infection is suspected much more frequently than it is confirmed, and that empiric antibiotics are prescribed frequently, and commonly continued even when microbiological confirmation of infection is lacking. Although many patients with infection receive inadequate therapy, we found no evidence that patients who receive more antibiotics do better. On the contrary, our findings suggest that prolonged administration of empiric therapy may be associated with adverse consequences. The inherent risks of ongoing empiric therapy, and the reluctance of clinicians to discontinue antibiotics once initiated, suggest that a more rigorous evaluation of empiric therapy is warranted.
References
Vincent JL (2003) Nosocomial infections in adult intensive-care units. Lancet 361:2068–2077
Esen S, Leblebicioglu H (2004) Prevalence of nosocomial infections at intensive care units in Turkey: a multicentre 1-day point prevalence study. Scand J Infect Dis 36:144–148
Rosenthal VD, Guzman S, Orellano PW (2003) Nosocomial infections in medical-surgical intensive care units in Argentina: attributable mortality and length of stay. Am J Infect Control 31:291–295
Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M (1995) The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. J Am Med Assoc 274:639–644
Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH (2002) Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 122:2115–2121
Warren DK, Shukla SJ, Olsen MA, Kollef MH, Hollenbeak CS, Cox MJ, Cohen MM, Fraser VJ (2003) Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med 31:1312–1317
Laupland KB, Kirkpatrick AW, Church DL, Ross T, Gregson DB (2004) Intensive-care-unit-acquired bloodstream infections in a regional critically ill population. J Hosp Infect 58:137–145
Brun-Buisson C, Roudot-Thoraval F, Girou E, Grenier-Sennelier C, Durand-Zaleski I (2003) The costs of septic syndromes in the intensive care unit and influence of hospital-acquired sepsis. Intensive Care Med 29:1464–1471
Blot S, Vandewoude K, Hoste E, Colardyn F (2003) Reappraisal of attributable mortality in critically ill patients with nosocomial bacteraemia involving Pseudomonas aeruginosa. J Hosp Infect 53:18–24
Eggimann P, Pittet D (2001) Infection control in the ICU. Chest 120:2059–2093
Richards MJ, Edwards JR, Culver DH, Gaynes RP (1999) Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med 27:887–892
Aarts MW, Marshall JC (2003) Empiric antibiotics in critical illness: Do they help or harm? In: Vincent JL (ed) Yearbook of intensive care and emergency medicine. Springer, Berlin, Heidelberg, New York, pp 219–228
Meyer E, Schwab F, Jonas D, Rueden H, Gastmeier P, Daschner FD (2004) Surveillance of antimicrobial use and antimicrobial resistance in intensive care units (SARI): 1. Antimicrobial use in German intensive care units. Intensive Care Med 30:1089–1096
Bergmans DC, Bonten MJ, Gaillard CA, van Tiel FH, van der GS, De Leeuw PW, Stobberingh EE (1997) Indications for antibiotic use in ICU patients: a one-year prospective surveillance. J Antimicrob Chemother 39:527–535
Bellomo R, Bersten AD, Boots RJ, Bristow PJ, Dobb GJ, Finfer SR, McArthur CJ, Richards B, Skowronski GA (1998) The use of antimicrobials in ten Australian and New Zealand intensive care units. The Australian and New Zealand Intensive Care Multicentre Studies Group Investigators. Anaesth Intensive Care 26(6):648–653
Kollef MH, Sherman G, Ward S, Fraser VJ (1999) Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462–474
Luna CM, Vujacich P, Niederman MS, Vay C, Gherardi C, Matera J, Jolly EC (1997) Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 111:676–685
Dupont H, Mentec H, Sollet JP, Bleichner G (2001) Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med 27:355–362
Harbarth S, Ferriere K, Hugonnet S, Ricou B, Suter P, Pittet D (2002) Epidemiology and prognostic determinants of bloodstream infections in surgical intensive care. Arch Surg 137:1353–1359
Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D (2003) Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med 115:529–535
Rello J, Ausina V, Ricart M, Castella J, Prats G (1993) Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest 104:1230–1235
Kollef MH, Fraser VJ (2001) Antibiotic resistance in the intensive care unit. Ann Intern Med 134:298–314
Yu VL, Singh N (2004) Excessive antimicrobial usage causes measurable harm to patients with suspected ventilator-associated pneumonia. Intensive Care Med 30:735–738
Fagon JY, Chastre J, Wolff M, Gervais C, Parer-Aubas S, Stephan F, Similowski T, Mercat A, Diehl JL, Sollet JP, Tenaillon A (2000) Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med 132:621–630
Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL (2000) Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 162:505–511
Marshall JC, Foster D, Vincent JL, Cook DJ, Cohen J, Dellinger RP, Opal S, Abraham E, Brett SJ, Smith T, Mehta S, Derzko A, Romaschin A (2004) Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC study. J Infect Dis 190:527–534
Aarts MW, Foster D, Derzko A, Marshall JC (2003) Antimicrobial therapy for suspected nosocomial ICU infection: aggressive empiric therapy does not improve outcome. Surg Infect 4:94–95
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985)APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ (1995) Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 23:1638–1652
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140
Kim JH, Gallis HA (1989) Observations on spiraling empiricism: its causes, allure, and perils, with particular reference to antibiotic therapy. Am J Med 87:201–206
Wunderink RG (1995) Ventilator-associated pneumonia. Failure to respond to antibiotic therapy. Clin Chest Med 16:173–193
Namias N, Harvill S, Ball S, McKenney MG, Salomone JP, Sleeman D, Civetta JM (1998) Empiric therapy of sepsis in the surgical intensive care unit with broad-spectrum antibiotics for 72 hours does not lead to the emergence of resistant bacteria. J Trauma 45:887–891
Heyland DK, Cook DJ, Marshall J, Heule M, Guslits B, Lang J, Jaeschke R (1999) The clinical utility of invasive diagnostic techniques in the setting of ventilator-associated pneumonia. Canadian Critical Care Trials Group. Chest 115:1076–1084
Ruiz M, Torres A, Ewig S, Marcos MA, Alcon A, Lledo R, Asenjo MA, Maldonaldo A (2000) Noninvasive versus invasive microbial investigation in ventilator- associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med 162:119–125
Sole Violan J, Fernandez JA, Benitez AB, Cardenosa Cendrero JA, Rodriguez de Castro F (2000) Impact of quantitative invasive diagnostic techniques in the management and outcome of mechanically ventilated patients with suspected pneumonia. Crit Care Med 28:2737–2741
Fagon JY, Chastre J, Hance AJ, Domart Y, Trouillet JL, Gibert C (1993) Evaluation of clinical judgment in the identification and treatment of nosocomial pneumonia in ventilated patients. Chest 103:547–553
Meduri GU, Mauldin GL, Wunderink RG, Leeper KV Jr, Jones CB, Tolley E, Mayhall G (1994) Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest 106:221–235
Aarts MW, Granton J, Cook DJ, Bohnen JMA, Marshall JC (in press). Empiric antimicrobial therapy in critical illness: results of an SIS Survey. Surg Infect
Acknowledgements
This work was supported in part by the physicians of Ontario through a grant from the Physicians' Services Incorporated Foundation. The MEDIC study was funded by Spectral Diagnostics Ltd. M.A.W. Aarts was the recipient of the Wyeth-Ayerst fellowship in clinical and evaluative sciences of the Surgical Infection Society. The work was presented in part at the 24th annual meeting of the Surgical Infection Society, San Antonio, Texas, April 2003.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Aarts, MA.W., Brun-Buisson, C., Cook, D.J. et al. Antibiotic management of suspected nosocomial ICU-acquired infection: Does prolonged empiric therapy improve outcome?. Intensive Care Med 33, 1369–1378 (2007). https://doi.org/10.1007/s00134-007-0723-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0723-y