Abstract
Objective
Intra-abdominal hypertension is an independent cause of multiorgan failure and directly effects other physiological measurements, making it an important factor in the management of critically ill patients, but no clinical studies have investigated the reproducibility of intra-abdominal pressure (IAP) measurement to ensure diagnostic accuracy. This study evaluated the intraobserver and interobserver variability of bladder pressure measurements.
Design and setting
Prospective, observational study in a university-based adult surgical intensive care unit.
Patients
Critically ill patients undergoing intra-abdominal pressure readings, measured by nursing staff.
Measurements and results
The study compared patient IAP measurements obtained by the same nurse (intraobserver variation) and between two different nurses (interobserver variation) in critical care patients with clinical indications for IAP monitoring. Data related to the nursing technique and performance were observed and collected for each IAP measurement obtained. Good correlation of bladder pressure measurements between the same and different individuals was found. Intraobserver and interobserver Pearson's correlations for measured IAP were 0.934 and 0.950, respectively. A unit protocol for IAP measurement standardization was modified based on observational data collected.
Conclusions
Intra-abdominal pressure can be accurately and reliably measured in critically ill patients by utilizing a standardized measurement device combined with a standardized clinical protocol.
Similar content being viewed by others
Introduction
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are increasingly recognized as significant sources of morbidity and mortality in critically ill patients [1]. However, detection of an increased intra-abdominal pressure (IAP) requires a reliable, reproducible method of measurement [2]. Noninvasive techniques to measure IAP include pressure transduction through a catheter in the stomach, urinary bladder, or rectum [3]. Of these, urinary bladder pressure measurement has emerged as the simplest and is considered the standard intermittent technique by the World Society on Abdominal Compartment Syndrome (WSACS; Consensus Definitions and Recommendations, January 2005, http://www.wsacs.org/; accessed 20 May 2005).
To date no in vivo studies have been published regarding the reproducibility of bladder pressure measurements. The goal of this study was to determine the reliability in terms of intra- and interobserver variability of bladder pressure measurements in a busy clinical intensive care unit (ICU) setting.
Materials and methods
Adult patients (or their legal representative) already undergoing IAP monitoring in a busy surgical ICU consented to participation in this institutional review board approved study. The study utilized a commercially available IAP monitoring system (AbViser, Wolfe Tory Medical, Salt Lake City, UT, USA) to obtain bladder pressure measurements. This kit interfaced with the ICU standard pressure transducer (Transpac IV, Abbott Labs, Chicago, IL, USA) which was zeroed at the pubic symphysis prior to each measurement set. Prior to clinical use of this device a carefully controlled bench-top investigation was conducted [4]. The bench-top data proved the device to be extremely accurate (± 1 mmHg) against a predefined fluid column. The ICU nursing staff received general in-service in IAP measurement acquisition prior to study implementation.
According to the study protocol, the patient was placed supine and, once calm (Riker score < 4), the IAP measurements were obtained. The primary nurse infused 50 ml saline fluid into the patient's bladder and the lowest end-expiratory pressure reading was recorded. This reading was considered measurement no. 1. After infusion drainage the same nurse repeated the process, obtaining measurement no. 2. Comparing these two values constituted the intraobserver variability portion of this study. A second nurse blinded to the original values then measured the IAP using the identical process. This measurement was considered measurement no. 3. Measurement no. 3 was compared to measurement no. 2 and constituted the interobserver variability data. A third nurse acted as the observer, documenting the measurement timing and technique as well as noting clinical factors that might contribute to data variation. This observer noted two factors that affected the quality of data collection: improper patient positioning and failure to allow adequate bladder drainage. These observations led to a more detailed protocol and an extension of data collection time from 5 to 8 min to allow complete bladder drainage between measurements.
Eighty-nine nurses participated in IAP measurement collection in 18 patients. There were 212 separate datasets obtained, with a range of 1–39 per patient, 12 of which were excluded for clinical concerns. Of the 176 intraobserver datasets 4 were excluded for exceeding the collection time of 8 min and of the 169 interobserver data sets 16 were excluded for exceeding the collection time. Descriptive statistics for the measures obtained by the first and second nurses are included in Table 1.
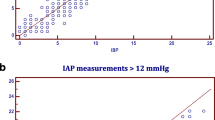
Data were analyzed using Stata 8.2 (College Station, TX, USA). Intraobserver and interobserver variability for the clinical phase were analyzed using Pearson's correlation coefficients and Bland–Altman comparisons and are illustrated with scatter plots and Bland–Altman plots [5, 6].
Results
Pearson's correlations for intraobserver and interobserver IAP measurement comparisons were 0.934 (p < 0.001) and 0.950 (p < 0.001), respectively, indicating low variability between measurements and therefore high reliability [5]. Intraobserver and interobserver scatterplots illustrate measurement consistency across observations (Figs. 1, 2). Bland–Altman analyses were also conducted, further demonstrating the consistency between measurements [6]. For intraobserver variability the mean difference between measured IAP was 0.570 mmHg, with a 95% confidence interval of 0.306–0.834 mmHg. Limits of agreement (or prediction interval) had a lower bound of −2.938 mmHg and an upper bound of 4.078 mmHg, with average measured pressure ranging from 4 to 25 mmHg (Fig. 3). For interobserver variability the mean difference between measured IAP was 0.000 mmHg, with a 95% confidence interval of −0.245 to 0.245 mmHg. Limits of agreement were −3.069 mmHg and 3.069 mmHg, with average measured pressure ranging from 3.00 to 23.50 mmHg (Fig. 4).
Discussion
Having reliable, reproducible data is imperative to properly manage critically ill patients whose physiology is in a state of rapid fluctuation. To prevent monitoring errors critical care textbooks emphasize the need to standardize monitoring processes, emphasizing the need for consistency from shift-to-shift and clinician-to-clinician to prevent data distortion [7]. This study found intraobserver and interobserver variations in IAP measurement are uncommon but not totally eliminated using a standardized IAP-monitoring kit and a strictly followed protocol. The cause of these occasional variations is likely physiological. Prior to conducting this clinical trial, we extensively evaluated the monitoring kit using a bench-top of IAP and found no intraobserver or interobserver variation, making device introduced data variation extremely unlikely [4]. De Potter et al. [8] have published a similar study, also confirming that data error is unlikely using a standardized device in a controlled bench-top setting. The most likely causes of data variability are patient activity, nursing procedural inconsistencies, and normal physiological variations of IAP. Documented study events causing data variability included coughing, resisting the ventilator, and agitation. These patient activities resulted in transient elevations in IAP presumably due to increased muscular activity and abdominal wall contractions. Other observed inconsistencies during measurements included incorrect patient positioning (upright position increases the pressure) and the timing of the bladder infusion.
The slight variations in IAP measurement demonstrated in this study are not surprising. Similar variations are seen during routine hemodynamic monitoring. As with IAP measurement, both central venous pressure (CVP) and pulmonary artery occlusion pressure (PAOP) show normal physiological fluctuation and require the patient to be in a nonagitated state and correctly positioned. One other investigator also noted similar IAP variations [9]. In an abstract report Peters et al. [9] compared intraobserver and interobserver variability of 50 nurses to the expertise of the chief investigator. Nurses' and chief investigator's correlation coefficients were r = 0.917 and r = 0.988, respectively. These authors conclude that measuring IAP according to recognized procedural steps results in reliable and reproducible data with little intraobserver and interobserver variability.
Transitioning bench-top experience to the bedside often presents unanticipated questions. In keeping with the protocol we adopted the WSACS recommendation to record IAP at end-expiration. However, it rapidly became apparent that end-expiratory pressure fluctuates from ventilation to ventilation and may actually be higher than inspiratory pressure in patients with forced exhalation. Rizvi et al. [10] suggest measuring airway pressure simultaneously to measuring other pressure parameters that are affected by airway pressure (PAOP, CVP, and IAP) to correct for forced exhalation. Although potentially confounding, adjustments for forced exhalation have not been routinely adopted for CVP or PAOP measurement and have not been suggested by WCACS for IAP measurement. To simplify and standardize this protocol we clarified our IAP measurement recommendation to be the lowest end-expiratory pressure seen over a 10- to 15-second assessment interval.
A second finding was that IAP fluctuations of 4 mmHg were relatively common. In this clinical setting we found that fluctuations over 2 mmHg occurred in 10.5% of paired measurements. Continuously recorded IAP suggests that minute to minute variation in IAP is very common [3]. We do not believe this makes the IAP measurement invalid, rather it suggests the need to obtain frequent data points to establish a trend rather than relying on a single measurement to make important management decisions (comparable to CVP, PAOP, and intracranial pressure).
This study has several limitations. The first is that only 18 patients were studied despite the fact that 212 separate measurements were obtained. However, because this study focuses on variability between nurses generating a data point and not on patient characteristics, using the same patient for repeated data generation reflects a valid variability potential. Based on the statistical analysis, it did not appear that further data collection would change the results or conclusions, and therefore no further data were collected. Another limitation of the study was failure to obtain either the second or the third measurement in every case (total measurement 212, 188, and 181 respectively). However, only data points that had a corresponding match and met the collection time criteria were evaluated.
This study has several clinical implications. The first is that by utilizing a standardized measurement device, combined with a clinical protocol to minimize confounding variables, IAP can be accurately and reliably measured in critically ill patients. The second is that minute to minute IAP fluctuations of more than 2 mmHg are common during routine IAP monitoring and are likely due to normal physiological variations. As with other physiological pressures, trends over time and in response to interventions should be emphasized. Finally, end-expiratory IAP may fluctuate by 1–3 mmHg during sequential cycles. We support following the WCACS recommendation to measure IAP at end-expiration but would add that in consideration of several respiratory cycles, the lowest IAP be measured. Simultaneous measurement of airway pressure may also be considered for improved accuracy in the setting of forced exhalation, but in a busy ICU setting this may not be practical.
References
Sugrue M (2005) Abdominal compartment syndrome. Curr Opin Crit Care 11:333–338
Sugrue M (2002) Intra-abdominal pressure: time for clinical practice guidelines? Intensive Care Med 28:389–391
Malbrain MLNG (2004) Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med 30:357–371
Wolfe TR, Kimball EJ (2005) Inter- and intra-observer variability does not occur with a new intra-abdominal pressure monitoring kit. ANZ J Surg 75:A1
Zar JH (1999) Biostatistical analyses. Prentice Hall, Upper Saddle River
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Darovic GO, Zbilut JB (2002) Fluid filled monitoring systems. In: Darovic GO (ed) Hemodynamic monitoring: invasive and noninvasive clinical applications. Saunders, Philadelphia, pp 113–131
DePotter TJ, Dits H, Malbrain MLNG (2005) Intra- and interobserver variability during in vitro validation of two novel methods for intra-abdominal pressure monitoring. Intensive Care Med 31:747–751
Peters K, Jones F, Sugrue M, Bauman A, Parr M, Balogh Z (2005) How reliable is intra-abdominal pressure measurement in intensive care? ANZ J Surg 75:A10
Rizvi K, deBoisblanc BP, Truwit JD, Dhillon G, Arroliga A, Fuchs BD, Guntupalli KK, Hite D, Hayden D (2005) Effect of airway pressure display on interobserver agreement in the assessment of vascular pressures in patients with acute lung injury and acute respiratory distress syndrome. Crit Care Med 33:98–103
Acknowledgements
E. J. K. is a concept and clinical consultant for Wolfe Tory Medical Inc., Salt Lake City, Utah, USA, which manufactures a commercially available kit for intra-abdominal pressure monitoring. To date all royalties received by him have been donated to a nursing education fund over which he has no personal oversight. T. R. W. is Vice President and Medical Director of Wolfe Tory Medical Inc., Salt Lake City, Utah, USA. The remaining authors have no financial involvement with any organization or entity with a financial interest in or in financial competition with the subject matter or materials discussed in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kimball, E.J., Mone, M.C., Wolfe, T.R. et al. Reproducibility of bladder pressure measurements in critically ill patients. Intensive Care Med 33, 1195–1198 (2007). https://doi.org/10.1007/s00134-007-0641-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0641-z