Abstract
Objective
To report the feasibility, complications, and outcomes of emergency extracorporeal life support (ECLS) in refractory cardiac arrests in medical intensive care unit (ICU).
Design and setting
Prospective cohort study in the medical ICU in a university hospital in collaboration with the cardiosurgical team of a neighboring hospital.
Patients
Seventeen patients (poisonings: 12/17) admitted over a 2-year period for cardiac arrest unresponsive to cardiopulmonary resuscitation (CPR) and advanced cardiac life support, without return of spontaneous circulation.
Interventions
ECLS femoral implantation under continuous cardiac massage, using a centrifugal pump connected to a hollow-fiber membrane oxygenator.
Measurements and results
Stable ECLS was achieved in 14 of 17 patients. Early complications included massive transfusions (n = 8) and the need for surgical revision at the cannulation site for bleeding (n = 1). Four patients (24%) survived at medical ICU discharge. Deaths resulted from multiorgan failure (n = 8), thoracic bleeding (n = 2), severe sepsis (n = 2), and brain death (n = 1). Massive hemorrhagic pulmonary edema during CPR (n = 5) and major capillary leak syndrome (n = 6) were observed. Three cardiotoxic-poisoned patients (18%, CPR duration: 30, 100, and 180 min) were alive at 1-year follow-up without sequelae. Two of these patients survived despite elevated plasma lactate concentrations before cannulation (39.0 and 20.0 mmol/l). ECLS was associated with a significantly lower ICU mortality rate than that expected from the Simplified Acute Physiology Score II (91.9%) and lower than the maximum Sequential Organ Failure Assessment score (> 90%).
Conclusions
Emergency ECLS is feasible in medical ICU and should be considered as a resuscitative tool for selected patients suffering from refractory cardiac arrest.
Similar content being viewed by others
Introduction
Cardiac arrest is a major worldwide public health concern [1]. Survival rate remains very low despite early access to emergency medical care, cardiopulmonary resuscitation (CPR), defibrillation, and advanced cardiac life support (ACLS). Extracorporeal life support (ECLS) is a well-recognized cardiac support technique in a growing number of primary cardiomyopathies and postoperative cardiac surgical cases [2]. Recently ECLS has been proposed as an ultimate rescue in prolonged cardiac arrest with failing conventional CPR [3, 4, 5]. However, the majority of out-of-hospital cardiac arrest victims are referred to intensive care units (ICUs) located in hospitals lacking a cardiothoracic surgery department, thus prohibiting ECLS initiation in combination with CPR. To date no alternative to prolonged CPR is routinely available. Failure to obtain spontaneous circulation with continuous external cardiac massage, even with stumpers, results in death. Our aim was to assess the feasibility of emergency ECLS as an implementation technique of pre- and intrahospital ACLS in the medical ICU. We report here our experience regarding possible indications, complications and outcome of ECLS as rescue therapy in patients with refractory cardiac arrest.
Materials and methods
Our medical and toxicological ICU is located in a university hospital (Hôpital Lariboisière) which has the largest emergency department in the Paris area but does not have a cardiovascular surgery department. Each year about 950 patients are admitted to our ICU, including 450 acute poisonings and 90 cardiac arrests. Thus, following the report of Massetti et al. [6] and Babatasi et al. [7], we requested the Comité d'Evaluation et de Diffusion des Innovations Technlogiques (CEDIT), an international and independent agency examining new health technology, to assess the value of ECLS in acute cardiotoxic poisonings. The CEDIT acknowledged that ECLS should be considered as the only alternative therapy in some poisoning cases, including refractory cardiac arrests and failures (http://cedit.aphp.fr/servlet/siteCeditGB?Destination=reco&numArticle=03.03/Re1/04). Thus based on the CEDIT recommendations and in order to offer optimal treatments to patients suffering from cardiac arrest, we have decided to develop ECLS technique in our ICU.
Patients whose conditions required continuous CPR over at least 45 min but in whom spontaneous circulation did not return because of refractory cardiac arrest were considered candidates for ECLS support. We specifically trained our medical team and nurses to perform ECLS. In addition, we collaborated with the cardiothoracic surgery department of a neighboring hospital (Hôpital Pitié Salpétrière) to obtain a consultant list of surgeons and perfusionists. When the decision to implant an ECLS in a cardiac arrest victim admitted to our ICU was taken by a senior intensivist of our team, a senior cardiac surgeon and a perfusionist from the neighboring hospital were immediately informed and came to our ICU as soon as possible, using a devoted ambulance.
Cannulation technique
ECLS was set up using the portable centrifugal pump (Rotaflow, Jostra-Maquet, Orléans, France) with a hollow-fiber membrane oxygenator (Quadrox) and a modified circuit (BEHQV 50600) suitable for use in ICU. The entire ECLS system and all instruments for vascular approach were put on mobile carts to facilitate their transportation. Whenever a call for ECLS was received, the circuit was quickly primed with normal saline. Peripheral femorofemoral cannulation was surgically set up by a team including a senior cardiac surgeon and a senior intensivist, as previously described using a modified Seldinger technique [3]. An additional distal limb perfusion was inserted to avoid severe leg ischemia. We used femoral 15- to 17-F arterial and 23- to 29-F venous Jostra cannulas according to the patient's size and 7-F catheters for distal limb perfusion. The tip of the arterial cannula was estimated to reside at the aortic-illiac junction, whereas the tip of the venous cannula was set in the right atrium. Pump flow was initially set at 3–4 l/min. Fluid repletion, norepinephrine, and epinephrine were used to treat cardiovascular shock aiming at obtaining a mean blood pressure of at least 60 mmHg. Venous cannula position was confirmed by echocardiography during the procedure while under CPR and confirmed radiologically when a stable ECLS flow was achieved.
Patient management during ECLS in ICU
ECLS was handled by our trained ICU personnel. Extracorporeal blood flow was adjusted to maintain adequate systemic blood flow and oxygen supply as monitored by mean arterial pressure, urine output, and plasma lactate concentrations. The doses of inotropic agents used before ECLS were progressively tapered following ECLS setup. Arterial pressure tracing was strictly monitored for reappearing pulsatile systemic blood flow, indicating residual left ventricular myocardial contractility facilitating left ventricular drainage. Dobutamine (10 μg/kg per minute) was infused to facilitate left ventricular decompression, minimizing the risks of pulmonary and left ventricle blood stasis as well as intracardiac clotting. In addition, a cardiac surgeon and a perfusionist were on call for management of emergencies that could not be handled by ICU personnel.
Blood samples were sent each 2 h to the laboratory for anticoagulation monitoring. Heparin was infused as early as possible to maintain a target activated clotting time 2.0–2.5 times higher than control at full flow. Because our patients were in critical condition and were expected to have severe impairment of coagulation, no intravenous heparin bolus was provided before cannulation. Patients were mechanically ventilated with 5–6 ml/kg tidal volume and 10 cmH2O positive end-expiratory pressure. The lowest possible fraction of inspired oxygen was used and guided by pulse oxymetry and radial arterial blood gas determination. Mild hypothermia was maintained during the first 24 h using a heater-cooler unit (Jostra-Maquet). Following the cannulation no further sedation was continued unless the patients showed signs of awakening. Echocardiography was used twice daily to assess venous cannula position, to exclude significant aortic regurgitation, and to assess progressive recovery of myocardial contractility. Serial routine biological tests and electrocardiogram (ECG) findings were daily checked. Neurological status was assessed using serial electroencephalograms during ECLS while the patient remained unresponsive.
The decision to discontinue ECLS support was based upon evidence of multiorgan failure, overwhelming sepsis, or severe neurological injury. The prerequisites to wean from ECLS were echocardiography assessment of myocardial function (left ventricular ejection fraction > 50%) and a radial PaO2/FIO2 ratio greater than 150 mmHg. The pump flow was tapered, given a clotting time more than 2.5 times higher than a control, to check for the absence of any deterioration in hemodynamic status. If the patient's cardiovascular status remained stable, ECLS was withdrawn by cardiac surgeons. If irreversible damage in myocardium function was diagnosed, the patient was transferred under ECLS, accompanied by the attending physician, a cardiac surgeon, and a perfusionist to the cardiosurgical ward of the neighboring hospital, to be considered for a ventricular assist device.
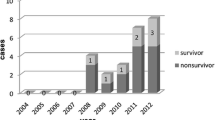
Patient selection and measurements
Between July 2003 to July 2005 we prospectively collected data on all 17 patients referred to our medical ICU with a witnessed prolonged out-of-hospital or intrahospital cardiac arrest treated with ECLS (12 women, 5 men; median age 47 years, range 27–57. All included patients had a presumed or identified heart-related cause of cardiac arrest (Fig. 1). Cardiac arrest occurred either out of the hospital (n = 9), including at home (n = 5), in the ambulance (n = 2), on the street (n = 1), and in a public place (n = 1), or in our hospital (n = 8), including in the emergency room (n = 2), the labor room (n = 1), and a medical ward (n = 5). Toxic causes of cardiac arrest (n = 12) included acute acebutolol (n = 3), flecainide (n = 2), chloroquine (n = 2), verapamil (n = 2), propranolol (n = 1), dextropropoxyphen (n = 1), and colchicine (n = 1) poisonings. Nontoxic causes included pulmonary embolism (n = 3), cardiomyopathy-related ventricular arrhythmia (n = 1), and amniotic embolism (n = 1). ECG showed persistent asystole (n = 13) or electromechanical dissociation with enlarged ventricular complexes (n = 4). Massive pulmonary edema (n = 5) occurred during CPR. Before connection to ECLS median arterial pH was 7.15 (10–90 percentiles 6.92–7.49), PaO2/FIO2 ratio 259 mmHg (51–516), PaCO2 38 mmHg (25–70), serum bicarbonate concentration 17.0 mmol/l (7.2–31.8), plasma lactate concentration 17.6 mmol/l (6.4–39.0), and serum creatinine concentration 128 μmol/l (86–339). The initial admission coagulation profile was the following: prothrombin time 36% (10–59%, expressed as a percentage of normal value), activated clotting time 2.0 (1.8–8.0) times higher than control, fibrinogen 1.70 g/l (0.50–2.47), and platelets 112 G/l (68–195).
CPR and advanced life-support were performed according to the standard guidelines currently recommended [8, 9]. Regarding out-of-hospital arrest, CPR was initiated by either bystanders or first responders and then continued at the scene and during patient transportation to hospital by the emergency medical service personnel (mobile medical team, ambulance crews, or firefighters). Cardiac arrest was considered refractory if no return of spontaneous circulation was obtained within 45 min despite continuous cardiac massage. This study was conducted according to the principles established in Helsinki. The study was approved by our institutional investigation review board. When possible, verbal consent was obtained from next of kin, provided with information regarding the purposes and risks of ECLS procedure.
ECLS feasibility in ICU was assessed with respect to time from cardiac arrest to ECLS initiation and to the percentage of successful procedures. ECLS was considered as successful if a mean blood pressure of 60 mmHg or higher and a flow rate of at least 3 l/min were obtained. Patients were evaluated for survival and morbidities. Outcome was evaluated according to survival at 24 h, ICU discharge, and ultimately at 1-year follow-up after hospital discharge. ECLS-attributable complications were documented. Capillary leak syndrome was defined as a generalized edema with persistent hypovolemia despite repeated vascular repletion.
Results of routine blood tests (including plasma lactate level), ECG, and toxicological screening were collected on admission (out-of hospital patients) or at the time of cardiac arrest (in-hospital patients), before connection to ECLS. Physiological variables measured at admission were used to calculate the Simplified Acute Physiology Score (SAPS) II [10]. The maximum Sequential Organ Failure Assessment (SOFA) score was defined by the highest score achieved during the entire ICU stay [11]. At 1-year follow up the Glasgow-Pittsburgh Cerebral Performance Category (CPC) was determined for the survivors [12].
Statistical analysis
For data analysis the patient population was split into two groups according to the cause of cardiac arrest: acute cardiotropic poisonings (“toxic group”) and other primary cardiac causes (“nontoxic group”). Results are presented as median and 10–90 percentiles. Comparisons between groups were performed using the Mann–Whitney U test for continuous variables or Fisher's exact test for categorical variables. Significance level was set at p ≤ 0.05. Statistical software (Statview, SAS, N.C., USA) was used for data analysis.
Results
Patients' median SAPS II was 79 (42–94), and maximum SOFA score 18.0 (17.0–20.0). CPR duration before ECLS initiation (120 min, 60–180) was prolonged, considering the delay needed for the cardiothoracic surgeon to arrive from the neighboring hospital to our ICU. Femoral arterial and venous cannulation was successful in all patients. Targeted extracorporeal flow was achieved in 14 of the 17 patients, with a duration of 40 h (3–154). Early complications included the need for surgical revision at the cannulation site for continuous bleeding (n = 1) and massive transfusions (n = 8, more than five packs in the first 24 h). Seven patients (41%) were alive at 24 h and 4 (24%) survived at ICU discharge. In our ICU deaths resulted from multiorgan failure (n = 8), catheter- and CPR-related thoracic bleeding (n = 2), severe sepsis (n = 2), and brain death (n = 1). A major capillary leak syndrome (n = 6) and a persistence of the hemorrhagic pulmonary edema (n = 5) were observed among the eight patients who died with multiorgan failure. In these patients ECLS termination was decided based on our conviction of irreversible signs of cellular damage, including probable brain death.
Among the four patients alive at medical ICU discharge the patient who suffered from severe cardiomyopathy, died in relation to hospital-acquired pneumonia 14 days after ECLS initiation and 2 days after her transfer under ECLS to the cardiothoracic surgery ward. Three poisoned patients (18%, out-of-hospital cardiac arrest, CPR duration 30, 100, and 180 min, respectively) were successfully weaned and left ICU at day 12, 13, and 14, respectively. The ingested toxicants [doses, plasma concentrations (therapeutic concentrations)] were flecainide [40 mg, 6.0 mg/l (0.2–0.6)] plus acebutolol [2 g, 1.8 mg/l (0.2–1.5)], acebutolol (40 g, 41.1 mg/l), and acebutolol (sustained-release preparation, 34 g, 40.2 mg/l), respectively. Two of the three survivors developed ventilator-acquired pneumonia successfully treated with antibiotics. No patient experienced limb ischemia. One survivor developed a scar infection. No significant technical incident occurred during ECLS, and no significant cannulation-related injuries of femoral vessels were reported. At 1-year follow-up, all three survivors were symptom-free, presenting no significant cardiac or neurological sequelae (CPC 1). ECLS was associated with a significantly lower ICU mortality rate than that expected from the resultant SAPS II (91.9%, p = 0.02) and lower than maximum SOFA score (> 90%).
Comparison of the “toxic” and “nontoxic” groups showed no significant differences except for the age (Table 1). The median CPR duration before ECLS was not significantly different according to survival at ICU discharge (110 min in the four survivors vs. 138 min in the 13 nonsurvivors, p = 0.4). Similarly, the median lactate level before ECLS initiation was not significantly different in relation to ICU survival (16.2 vs. 17.6 mmol/l, p = 0.8). Interestingly, the flecainide/acebutolol-poisoned patient survived with a very elevated plasma lactate concentration on presentation (39 mmol/l).
Discussion
Over a 2-year period 17 witnessed and CPR-refractory cardiac arrests were managed using ECLS in our ICU. We recorded a long-term survival of 18%. Our results clearly suggest that ECLS implementation using femoral cannulation is feasible in a medical ICU, with limited complications and hopeful results. The delay to ECLS implementation [120 min (60–180) median duration vs. 105 ± 44 min average time of cardiac massage] and the patient survival rate (3/17 vs. 8/40) were comparable with those previously reported [3]. However, the hemorrhagic risk of this invasive technique remains an important concern as it may offset ECLS potential benefits. In three of our patients massive transfusions were related to local or thoracic bleedings (cardiac massage and/or prehospital attempts to insert a venous catheter); in five others they resulted from hemorrhagic pulmonary edema, major capillary leaking syndrome, and venous congestion.
Experience with cardiopulmonary bypass during cardiac arrest remains limited with variable survival rates (0–64%) [3, 4, 6, 7, 13, 14, 15, 16, 17, 18]. Differences in outcome are related to the small number of patients, causes of cardiac arrest, variable delay until onset of circulatory support, and varying experience with this technique. In our series, in contrast to that of Massetti et al. [3], definitive survival was obtained only in poisonings. Cardiotoxic intoxication and severe hypothermia are the two accepted indications for ECLS in cardiac arrest [3, 4]. Our results clearly support that irreversibility of the underlying cardiac pathology and cerebral ischemic injury after prolonged CPR is a serious limitation for emergency ECLS.
Cardiotoxic poisoning-related death occurs in relation to sudden ventricular tachycardia or fibrillation as well as myocardial contractility incompetence [19]. In some cases return of spontaneous circulation may be achieved with CPR alone, suggesting a reversible pathophysiology [20]. However, massive drug ingestions may be associated with refractory cardiac failure, which reversibility makes ECLS promising despite prolonged arrest (grade C recommendation) [1, 3, 6, 7, 21]. In selected patients, cardiopulmonary bypass may be life-saving, provided that the patient has not sustained hypoxic cerebral damage due to prolonged no or low flow prior to its initiation. Once implemented, the purpose of ECLS is to take over heart function until recovery can occur, thus minimizing myocardial work, improving organ perfusion, and maintaining the renal and biliary elimination of the toxicant [22, 23, 24].
Long-term survival was shown to be possible after emergency ECLS in patients with underlying cardiocirculatory diseases amenable to immediate corrective intervention, such as angioplasty, surgery, and transplantation [4]. In patients experiencing myocardial infarction with either cardiac arrest or refractory shock ECLS afforded a better chance of survival [5]. Up to 50% survival rates have been obtained in patients resuscitated in the controlled environment of the catheter laboratory [25]. In our series no myocardial infarction was included. However, three patients suffered from pulmonary embolism with refractory cardiac arrest despite thrombolysis and one from amniotic fluid embolism. All cases were evidenced by necropsy. Mortality related to pulmonary embolism remains high [26]. Similarly, amniotic fluid embolism is associated with poor maternal and neonatal survival, when cardiac arrest occurs [27].
The decision to withhold resuscitation is generally based upon presumed prolonged anoxia, with existing advisory rules for termination [28, 29]. In the majority of victims transportation to emergency departments remains inappropriate, futile, and ethically unacceptable [9]. However, in immediately resuscitated victims our results suggest the necessity of early medical recognition of potential reversible causes, such as poisoning, for a rapid transport with continuing CPR to ECLS-equipped hospitals. In these candidates for ECLS rescue the quality of prehospital CPR remains, as for all cardiac arrest victims [30], the critical step for neurological outcome.
Survival from cardiac arrest is critically related to the delay of initiation of basic and ACLS [31]. High lactate levels (> 16.3 mmol/l) were generally associated with unfavorable neurological recovery, with a 100% specificity [32]. However, based on our observations this value assessed to predict outcome appears questionable in immediately resuscitated poisoning-related cardiac arrest victims. In our series at least two poisoned patients with high lactate levels (20.0 and 39.0 mmol/l) completely recovered. Moreover, there were no significant differences in lactate concentration on admission according to survival. However, although a weak association between cardiac arrest duration and admission lactate levels has been shown [32], functional neurological recovery is more likely to be unfavorable with increasing concentrations. In a multivariate analysis lactate level at 48 h was an independent predictor for mortality and unfavorable neurological outcome [33]. In our two survivors ECLS allowed rapid decrease in lactate concentrations (2.2 and 3.2 mmol/l at 48 h, respectively).
Long delays to return of spontaneous circulation are compatible with good cognitive outcome, confirming that duration should not limit CPR efforts [34]. Good outcome-contributing factors include immediate and skilled basic and ACLS, ventricular fibrillation as the primary and persistent rhythm, age under 70 years, absence of previous medical diseases, and highly aggressive postresuscitation care [35]. However, although guidelines exist to avoid futile efforts [36], early criteria of no recovery despite ECLS and potential interest of the recently marketed CPR assist devices remain to be determined. Moreover, future research should evaluate ECLS to provide both systemic cooling and hemodynamic support in cardiac arrest.
Several limitations to this single center study exist. The number of patients remained insufficient to yield any persuasive statistically significant findings. Otherwise, due to the specificity of our center, there is a selection bias regarding the number of poisonings. Inclusion of various causes of cardiac arrest with various degrees of heart dysfunction reversibility also limits definitive comparisons. Moreover, decision to stop CPR and not to transport patients under cardiac massage to our ICU was independent of our team, making difficult any estimation of the proportion of refractory cardiac arrest victims that should have benefited from ECLS. Another limitation of ECLS performed in a medical ward would be its yearly incidence. The threshold bellow which ECLS practice would be of concern is unknown.
In conclusion, ECLS instituted in medical ICU in close collaboration with a cardiosurgical ward is feasible and effective for the hemodynamic resuscitation of ACLS-unresponsive cardiac arrest. Based on our experience, ECLS should be considered as an emergency resuscitative tool, providing the ability for rapid intervention of cardiac surgeon to control ECLS-related complications. Thus ECLS remains to date an investigational and compassionate treatment until further studies assess its efficiency in refractory cardiac arrest from nontoxic origin.
References
Stiell IG, Wells GA, Field B, Spaite DW, Nesbitt LP, De Maio VJ, Nichol G, Cousineau D, Blackburn J, Munkley D, Luinstra-Toohey L, Campeau T, Dagnone E, Lyver M, Ontario Prehospital Advanced Life Support Study Group (2004) Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med 351:647–656
Conrad SA, Rycus PT, Dalton H (2005) Extracorporeal life support registry report 2004. ASAIO J 51:4–10
Massetti M, Tasle M, Le Page O, Deredec R, Babatasi G, Buklas D, Thuaudet S, Charbonneau P, Hamon M, Grollier G, Gerard JL, Khayat A (2005) Back from irreversibility: extracorporeal life support for prolonged cardiac arrest. Ann Thorac Surg 79:178–183
Schwarz B, Mair P, Margreiter J, Pomaroli A, Hoermann C, Bonatti J, Lindner KH (2003) Experience with percutaneous venoarterial cardiopulmonary bypass for emergency circulatory support. Crit Care Med 31:758–764
Chen JS, Ko WJ, Yu HY, Lai LP, Huang SC, Chi NH, Tsai CH, Wang SS, Lin FY, Chen YS (2006) Analysis of the outcome for patients experiencing myocardial infarction and cardiopulmonary resuscitation refractory to conventional therapies necessitating extracorporeal life support rescue. Crit Care Med 34:950–957
Massetti M, Bruno P, Babatasi G, Neri E, Khayat A (2000) Cardiopulmonary bypass and severe drug intoxication. J Thorac Cardiovasc Surg 120:424–425
Babatasi G, Massetti M, Verrier V, Lehoux P, Le Page O, Bruno PG, Khayat A (2001) [Severe intoxication with cardiotoxic drugs: value of emergency percutaneous cardiocirculatory assistance]. Arch Mal Coeur Vaiss 94:1386–1392
Cummins RO, Ornato JP, Thies WH, Pepe PE (1991) Improving survival from sudden cardiac arrest: the chain of survival concept. Circulation 83:1832–1847
International Liaison Committee on Resuscitation (2005) Part 4: Advanced life support. Resuscitation 67:213–247
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800
Booth CM, Boone RH, Tomlinson G, Detsky AS (2004) Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA 291:870–879
Nagao K, Hayashi N, Kanmatsuse K, Arima K, Ohtsuki J, Kikushima K, Watanabe I (2000) Cardiopulmonary cerebral resuscitation using emergency cardiopulmonary bypass, coronary reperfusion therapy and mild hypothermia in patients with cardiac arrest outside the hospital. J Am Coll Cardiol 36:776–783
Younger JG, Schreiner RJ, Swaniker F, Hirschl RB, Chapman RA, Bartlett RH (1999) Extracorporeal resuscitation of cardiac arrest. Acad Emerg Med 6:700–707
Martin GB, Rivers EP, Paradis NA, Goetting MG, Morris DC, Nowak RM (1998) Emergency department cardiopulmonary bypass in the treatment of human cardiac arrest. Chest 113:743–751
Mair P, Hoermann C, Moertl M, Bonatti J, Falbesoner C, Balogh D (1996) Percutaneous venoarterial extracorporeal membrane oxygenation for emergency mechanical circulatory support. Resuscitation 33:29–34
Hartz R, LoCicero J 3rd, Sanders JH Jr, Frederiksen JW, Joob AW, Michaelis LL (1990) Clinical experience with portable cardiopulmonary bypass in cardiac arrest patients. Ann Thorac Surg 50:437–441
Reichman RT, Joyo CI, Dembitsky WP, Griffith LD, Adamson RM, Daily PO, Overlie PA, Smith SC Jr, Jaski BE (1990) Improved patient survival after cardiac arrest using a cardiopulmonary support system. Ann Thorac Surg 49:101–104
Watson WA, Litovitz TL, Rodgers GC Jr, Klein-Schwartz W, Reid N, Youniss J, Flanagan A, Wruk KM (2005) 2004 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 23:589–666
Paredes VL, Rea TD, Eisenberg MS, Cobb LA, Copass MK, Cagle A, Martin TG (2004) Out-of-hospital care of critical drug overdoses involving cardiac arrest. Acad Emerg Med 11:71–74
Albertson TE, Dawson A, de Latorre F, Hoffman RS, Hollander JE, Jaeger A, Kerns WR 2nd, Martin TG, Ross MP, American Heart Association, International Liaison Committee on Resuscitation (2001) TOX-ACLS: toxicologic-oriented advanced cardiac life support. Ann Emerg Med 37(Suppl 4):S78–S90
Banner W Jr (1996) Risks of extracorporeal membrane oxygenation: is there a role for use in the management of the acutely poisoned patient? J Toxicol Clin Toxicol 34:365–371
Purkayastha S, Bhangoo P, Athanasiou T, Casula R, Glenville B, Darzi AW, Henry JA (2006) Treatment of poisoning induced cardiac impairment using cardiopulmonary bypass: a review. Emerg Med J 23:246–250
Mégarbane B, Leprince P, Deye N, Guerrier G, Résière D, Bloch V, Baud FJ (2006) Extracorporeal life support in a case of acute carbamazepine poisoning with life-threatening refractory myocardial failure. Intensive Care Med 32:1409–1413
Mooney MR, Arom KV, Joyce LD, Mooney JF, Goldenberg IF, von Rueden TJ, Emery RW (1991) Emergency cardiopulmonary bypass support in patients with cardiac arrest. J Thorac Cardiovasc Surg 101:450–454
Kurkciyan I, Meron G, Sterz F, Janata K, Domanovits H, Holzer M, Berzlanovich A, Bankl HC, Laggner AN (2000) Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med 160:1529–1535
Moore J, Baldisseri MR (2005) Amniotic fluid embolism. Crit Care Med 33:S279–S85
Horsted TI, Rasmussen LS, Lippert FK, Nielsen SL (2004) Outcome of out-of-hospital cardiac arrest—why do physicians withhold resuscitation attempts? Resuscitation 63:287–293
Morrison LJ, Visentin LM, Kiss A, Theriault R, Eby D, Vermeulen M, Sherbino J, Verbeek PR (2006) Validation of a rule for termination of resuscitation in out-of-hospital cardiac arrest. N Engl J Med 355:478–487
Wik L, Kramer-Johansen J, Myklebust H, Sorebo H, Svensson L, Fellows B, Steen PA (2005) Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA 293:299–304
Vukmir RB (2006) Survival from prehospital cardiac arrest is critically dependent upon response time. Resuscitation 69:229–234
Mullner M, Sterz F, Domanovits H, Behringer W, Binder M, Laggner AN (1997) The association between blood lactate concentration on admission, duration of cardiac arrest, and functional neurological recovery in patients resuscitated from ventricular fibrillation. Intensive Care Med 23:1138–1143
Kliegel A, Losert H, Sterz F, Holzer M, Zeiner A, Havel C, Laggner AN (2004) Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine (Baltimore) 83:274–279
Van Alem AP, de Vos R, Schmand B, Koster RW (2004) Cognitive impairment in survivors of out-of-hospital cardiac arrest. Am Heart J 148:416–421
Cooper S, Macnaughton P (2001) Prolonged resuscitation: a case report. Resuscitation 50:349–351
Hill JG, Bruhn PS, Cohen SE, Gallagher MW, Manart F, Moore CA, Seifert PE, Askari P, Banchieri C (1992) Emergent applications of cardiopulmonary support: a multiinstitutional experience. Ann Thorac Surg 54:699–704
Acknowledgements
The authors acknowledge Dr. Rebecca Gracia, PharmD, DABAT, from the North Texas Poison Center, Dallas, Texas, USA, for her helpful review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-006-0569-3
Rights and permissions
About this article
Cite this article
Mégarbane, B., Leprince, P., Deye, N. et al. Emergency feasibility in medical intensive care unit of extracorporeal life support for refractory cardiac arrest. Intensive Care Med 33, 758–764 (2007). https://doi.org/10.1007/s00134-007-0568-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0568-4