Abstract
Objective
Drug dosing during continuous venovenous hemofiltration (CVVH) is based partly upon the CVVH clearance (ClCVVH) of the drug. ClCVVH is the product of the sieving coefficient (SC) and ultrafiltration rate (Quf). Although it has been suggested that the SC can be replaced by the fraction of a drug not bound to protein (Fup), the Fup values as reported in the literature may not reflect the protein binding in critically ill patients with renal failure. We compared the observed ClCVVH (SC × Quf) with the estimated ClCVVH (estimated FUP × Quf) and determined the effect on the maintenance dose multiplication factor (MDMF).
Design and setting
Clinical study in a mixed ICU in a university hospital.
Patients
45 oligoanuric patients on CVVH (2 l/h).
Interventions
Timed blood and ultrafiltrate samples.
Measurements and results
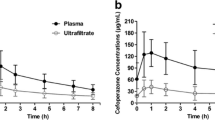
Amoxicillin, ceftazidime, ciprofloxacin, fluconazole, metronidazole, and vancomycin were easily filtered (mean SC > 0.7) but not flucloxacillin (mean SC 0.3). Predicted and observed ClCVVH corresponded only for fluconazole and metronidazole. The difference between observed and predicted MDMF was small for all drugs, with the exception of ceftazidime (mean 0.25, 95% CI −0.96 to 1.48) and vancomycin (0.05, −1.34 to 1.45). However, this difference was clinically relevant only for vancomycin, because of its narrow therapeutic index.
Conclusions
Dosing based on predicted CVVH removal provides an as reliable estimate than that based on observed CVVH removal except for those antibiotics that have both a narrow therapeutic index and a predominantly renal clearance (e.g., vancomycin).


Similar content being viewed by others
References
Mehrotra R, De Gaudio R, Palazzo M (2004) Antibiotic pharmacokinetic and pharmacodynamic considerations in critical illness. Intensive Care Med 30:2145–2156
Subach RA, Marx MA (1998) Drug dosing in acute renal failure: the role of renal replacement therapy in altering drug pharmacokinetics. Adv Ren Replace Ther 5:141–147
Reetze-Bonorden P, Bohler J, Keller E (1993) Drug dosage in patients during continuous renal replacement therapy. Pharmacokinetic and therapeutic considerations. Clin Pharmacokinet 24:362–379
Lau AH, Kronfol NO (1994) Determinants of drug removal by continuous hemofiltration. Int J Artif Organs 17:373–378
Bohler J, Donauer J, Keller F (1999) Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int Suppl 72:S24–S28
Golper TA, Wedel SK, Kaplan AA, Saad AM, Donta ST, Paganini EP (1985) Drug removal during continuous arteriovenous hemofiltration: theory and clinical observations. Int J Artif Organs 8:307–312
Bugge JF (2001) Pharmacokinetics and drug dosing adjustments during continuous venovenous hemofiltration or hemodiafiltration in critically ill patients. Acta Anaesthesiol Scand 45:929–934
Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G (2000) Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 356:26–30
Bouman C, Kan H, Schultz M, Vroom M (2005) The removal of antibiotics during continuous venovenous hemofiltration (CVVH) in critically ill patients with acute renal failure: measured versus estimated CVVH clearance (abstract). Crit Care 9:14
Clark WR, Ronco C (1999) CRRT efficiency and efficacy in relation to solute size. Kidney Int Suppl 72:S3–S7
Schetz M, Ferdinande P, Van den BG, Verwaest C, Lauwers P (1995) Pharmacokinetics of continuous renal replacement therapy. Intensive Care Med 21:612–620
Boereboom FT, Ververs FF, Blankestijn PJ, Savelkoul TJ, van Dijk A (1999) Vancomycin clearance during continuous venovenous haemofiltration in critically ill patients. Intensive Care Med 25:1100–1104
Matzke GR, O'Connell MB, Collins AJ, Keshaviah PR (1986) Disposition of vancomycin during hemofiltration. Clin Pharmacol Ther 40:425–430
Yagasaki K, Gando S, Matsuda N, Kameue T, Ishitani T, Hirano T, Iseki K (2003) Pharmacokinetics and the most suitable dosing regimen of fluconazole in critically ill patients receiving continuous hemodiafiltration. Intensive Care Med 29:1844–1848
Joos B, Schmidli M, Keusch G (1996) Pharmacokinetics of antimicrobial agents in anuric patients during continuous venovenous haemofiltration. Nephrol Dial Transplant 11:1582–1585
Matzke GR, Frye RF, Joy MS, Palevsky PM (2000) Determinants of ceftazidime clearance by continuous venovenous hemofiltration and continuous venovenous hemodialysis. Antimicrob Agents Chemother 44:1639–1644
Traunmuller F, Schenk P, Mittermeyer C, Thalhammer-Scherrer R, Ratheiser K, Thalhammer F (2002) Clearance of ceftazidime during continuous venovenous haemofiltration in critically ill patients. J Antimicrob Chemother 49:129–134
Bellmann R, Egger P, Gritsch W, Bellmann-Weiler R, Joannidis M, Dunzendorfer S, Wiedermann C (2002) Pharmacokinetics of ciprofloxacin in patients with acute renal failure undergoing continuous venovenous haemofiltration: influence of concomitant liver cirrhosis. Acta Med Austriaca 29:112–116
Malone RS, Fish DN, Abraham E, Teitelbaum I (2001) Pharmacokinetics of levofloxacin and ciprofloxacin during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 45:2949–2954
Muhl E, Martens T, Iven H, Rob P, Bruch HP (2000) Influence of continuous veno-venous haemodiafiltration and continuous veno-venous haemofiltration on the pharmacokinetics of fluconazole. Eur J Clin Pharmacol 56:671–678
Valtonen M, Tiula E, Neuvonen PJ (1997) Effect of continuous venovenous haemofiltration and haemodiafiltration on the elimination of fluconazole in patients with acute renal failure. J Antimicrob Chemother 40:695–700
Shah M, Quigley R (2000) Rapid removal of vancomycin by continuous veno-venous hemofiltration. Pediatr Nephrol 14:912–915
Uchino S, Cole L, Morimatsu H, Goldsmith D, Bellomo R (2002) Clearance of vancomycin during high-volume haemofiltration: impact of pre-dilution. Intensive Care Med 28:1664–1667
Meyer B, Ahmed eG, Delle KG, Locker GJ, Heinz G, Jaeger W, Thalhammer F (2003) How to calculate clearance of highly protein-bound drugs during continuous venovenous hemofiltration demonstrated with flucloxacillin. Kidney Blood Press Res 26:135–140
Cigarran-Guldris S, Brier ME, Golper TA (1991) Tobramycin clearance during simulated continuous arteriovenous hemodialysis. Contrib Nephrol 93:120–123
Matzke GR, Frye RF, Joy MS, Palevsky PM (2000) Determinants of ceftriaxone clearance by continuous venovenous hemofiltration and hemodialysis. Pharmacotherapy 20:635–643
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bouman, C.S.C., van Kan, H.J.M., Koopmans, R.P. et al. Discrepancies between observed and predicted continuous venovenous hemofiltration removal of antimicrobial agents in critically ill patients and the effects on dosing. Intensive Care Med 32, 2013–2019 (2006). https://doi.org/10.1007/s00134-006-0397-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0397-x