Abstract
Objective
To determine the workplace concentrations of NO and NO2 in and around a paediatric incubator during inhaled NO (iNO) treatment and during an accidental emptying of NO cylinders into room air.
Design
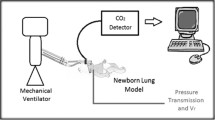
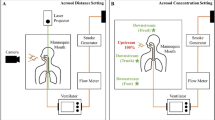
We simulated iNO–nasal CPAP treatment in order to assess the impact on the occupational environment. Furthermore, two full NO cylinders for therapy, 1,000 ppm, 20 litres, 150 bar and 400 ppm, 10 litres, 150 bar, were emptied as rapidly as possible into an intensive care unit (ICU) room.
Setting
University hospital ICU.
Measurements and results
To correctly gauge the contribution from iNO–CPAP we constructed a system measuring breathing zone and room ventilation inlet-outlet values during a 10-ppm iNO treatment of a simulated infant. Maximal breathing zone values were 17.9 ± 7.0 (mean ± 95% CI) ppb for NO and 25.2 ± 4.8 ppb for NO2. If room inlet values were subtracted, the contributions to breathing zone values emanating from iNO–CPAP were 14.8 ± 4.6 ppb for NO and 14.6 ± 4.6 ppb for NO2. At the ventilation outlet the maximal contributions were 4.2 ± 2.9 ppb NO and 9.6 ± 4.3 ppb NO2. During rapid total release of a gas cylinder in the ICU room, simulating an accident, we found transient NO levels comparable to the high therapeutic dosing range, but only low NO2 levels.
Conclusions
Neither 8-h time-weighted average (TWA) nor 15 min short-term exposure limits (STEL) were exceeded during normal operation or during a simulated accident. The contribution of nitrogen oxides from treatment to workplace air were minor compared to those from ambient air.




Similar content being viewed by others
References
Stevens T, Blennow M, Soll R (2004) Early surfactant administration with brief ventilation vs selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 3:CD003063
Soll RF, Morley CJ (2001) Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev CD000510
Jonsson B, Katz-Salamon M, Faxelius G, Broberger U, Lagercrantz H (1997) Neonatal care of very-low-birthweight infants in special-care units and neonatal intensive-care units in Stockholm. Early nasal continuous positive airway pressure versus mechanical ventilation: gains and losses. Acta Paediatr Suppl 419:4–10
Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrom K, Jacobsen T (1994) Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med 331:1051–1055
Polin RA, Sahni R (2002) Newer experience with CPAP. Semin Neonatol 7:379–389
Van Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, Susser M, Paneth N, Leviton A (2000) Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics 105:1194–1201
Lindwall R, Frostell CG, Lonnqvist PA (2002) Delivery characteristics of a combined nitric oxide nasal continuous positive airway pressure system. Paediatr Anaesth 12:530–536
Lindwall R, Blennow M, Svensson M, Jonsson B, Berggren-Bostrom E, Flanby M, Lonnqvist PA, Frostell C, Norman M (2005) A pilot study of inhaled nitric oxide in preterm infants treated with nasal continuous positive airway pressure for respiratory distress syndrome. Intensive Care Med 31:959–964
Andrews P, Azoulay E, Antonelli M, Brochard L, Brun-Buisson C, Dobb G, Fagon JY, Gerlach H, Groeneveld J, Mancebo J, Metnitz P, Nava S, Pugin J, Pinsky M, Radermacher P, Richard C, Tasker R (2006) Year in review in intensive care medicine, 2005. III. Nutrition, pediatric and neonatal critical care, and experimental. Intensive Care Med 32:490–500
McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, Crapo JD, Grubb PH, Shaul PW (2005) Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 288:L450–L459
Ballard PL, Gonzales LW, Godinez RI, Godinez MH, Savani RC, McCurnin DC, Gibson LL, Yoder BA, Kerecman JD, Grubb PH, Shaul PW (2006) Surfactant composition and function in a primate model of infant chronic lung disease: effects of inhaled nitric oxide. Pediatr Res 59:157–162
Jones C (1998) Inhaled nitric oxide: are the safety issues being addressed? Intensive Crit Care Nurs 14:271–275
Markhorst DG, Leenhoven T, Uiterwijk JW, Meulenbelt J, van Vught AJ (1996) Occupational exposure during nitric oxide inhalational therapy in a pediatric intensive care setting. Intensive Care Med 22:954–958
Putman E, van Golde LM, Haagsman HP (1997) Toxic oxidant species and their impact on the pulmonary surfactant system. Lung 175:75–103
Muller B, Seifart C, Barth PJ (1998) Effect of air pollutants on the pulmonary surfactant system. Eur J Clin Invest 28:762–777
Mourgeon E, Levesque E, Duveau C, Law-Koune JD, Charbit B, Ternissien E, Coriat P, Rouby JJ (1997) Factors influencing indoor concentrations of nitric oxide in a Parisian intensive care unit. Am J Respir Crit Care Med 156:1692–1695
Kinsella JP, Griebel J, Schmidt JM, Abman SH (2002) Use of inhaled nitric oxide during interhospital transport of newborns with hypoxemic respiratory failure. Pediatrics 109:158–161
Schedin U, Frostell CG, Gustafsson LE (1999) Formation of nitrogen dioxide from nitric oxide and their measurement in clinically relevant circumstances. Br J Anaesth 82:182–192
Moa G, Nilsson K, Zetterstrom H, Jonsson LO (1988) A new device for administration of nasal continuous positive airway pressure in the newborn: an experimental study. Crit Care Med 16:1238–1242
Merilainen PT (1990) A differential paramagnetic sensor for breath-by-breath oximetry. J Clin Monit 6:65–73
Phillips ML, Hall TA, Sekar K, Tomey JL (1999) Assessment of medical personnel exposure to nitrogen oxides during inhaled nitric oxide treatment of neonatal and pediatric patients. Pediatrics 104:1095–1100
Qureshi MA, Shah NJ, Hemmen CW, Thill MC, Kruse JA (2003) Exposure of intensive care unit nurses to nitric oxide and nitrogen dioxide during therapeutic use of inhaled nitric oxide in adults with acute respiratory distress syndrome. Am J Crit Care 12:147–153
Johansson C, Hadenius A, Johansson PÅ, Jonson T (1999) SHAPE, The Stockholm Study on Health Effects of Air Pollution and their Economic Consequences Part I: NO2 and Particulate Matter in Stockholm. 41:1–71
Benzing A, Loop T, Mols G, Geiger K (1999) Unintended inhalation of nitric oxide by contamination of compressed air: physiologic effects and interference with intended nitric oxide inhalation in acute lung injury. Anesthesiology 91:945–950
Lee KH, Tan PS, Rico P, Delgado E, Kellum JA, Pinsky MR (1997) Low levels of nitric oxide as contaminant in hospital compressed air: physiologic significance? Crit Care Med 25:1143–1146
Pinsky MR, Genc F, Lee KH, Delgado E (1997) Contamination of hospital compressed air with nitric oxide: unwitting replacement therapy. Chest 111:1759–1763
Tan PS, Genc F, Delgado E, Kellum JA, Pinsky MR (2002) Nitric oxide contamination of hospital compressed air improves gas exchange in patients with acute lung injury. Intensive Care Med 28:1064–1072
Berglund M, Boström CE, Bylin G, Ewetz L, Gustafsson LE, Moldeus P, Pershagen G, Victorin K (1993) Health risk evaluation of nitrogen oxides. Exposure. Scand J Work Environ Health 19 Suppl 2:1–72
Kraft M, Eikmann T, Kappos A, Kunzli N, Rapp R, Schneider K, Seitz H, Voss JU, Wichmann HE (2005) The German view: effects of nitrogen dioxide on human health – derivation of health-related short-term and long-term values. Int J Hyg Environ Health 208:305–318
Schedin U, Norman M, Gustafsson LE, Jonsson B, Frostell C (1997) Endogenous nitric oxide in the upper airways of premature and term infants. Acta Paediatr 86:1229–1235
Acknowledgements
This study was supported by the European Space Agency, the Swedish Science Council, the Swedish Heart-Lung Foundation, Karolinska Institutet and the Stockholm County Council. INO Therapeutics (through Berit Lindh, Nordic manager) kindly provided study gases. The authors are indebted to Dr C-J. Wickerts, head of the ICU at Danderyd University Hospital, for access to ICU facilities as a setting for these studies. The author C.F. wishes to disclose a conflict of interest as he has a financial interest in the clinical use of inhaled nitric oxide, and L.G. is a minority shareholder in Aerocrine AB (publ.), a company that markets instruments for measurements of exhaled nitric oxide, and has given consultancy to gas industry concerning the use of nitric oxide.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindwall, R., Svensson, M.E., Frostell, C.G. et al. Workplace NO and NO2 during combined treatment of infants with nasal CPAP and NO. Intensive Care Med 32, 2034–2041 (2006). https://doi.org/10.1007/s00134-006-0393-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0393-1