Abstract
Objective
To investigate whether the compartment pressure of the rectus sheath (CPRS) reflects the intra-abdominal pressure (IAP) under various conditions of intra-abdominal hypertension (IAH).
Design and setting
Prospective experimental study with in vivo pressure measurements at the Institute for Clinical and Experimental Surgery, University of Saarland.
Animals
Sprague-Dawley rats.
Interventions
Stepwise increase and decrease in IAP with continuous measurement of the correspondent CPRS.
Measurements and results
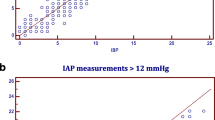
Physiological IAP (2 mmHg) and CPRS (6 mmHg) showed a statistically significant difference. Stepwise elevation in IAP was associated with a simultaneous increase in CPRS. Accordingly, stepwise decompression of IAP resulted in a stepwise decrease in CPRS. Under both conditions Bland-Altman analysis comparing IAP to correspondent CPRS showed a very good agreement for IAP at or above 12 mmHg. In addition, closure of the overlaying subcutaneous tissue and skin did not affect CPRS or its correlation with IAP.
Conclusions
CPRS accurately reflects IAP for IAP of 12 mmHg or higher. Thus CPRS measurements may represent a novel approach for diagnosis and monitoring of IAH.
Similar content being viewed by others
Introduction
In recent years intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) have been recognized in trauma and visceral surgery patients. The acceptance of damage control laparotomy in critically injured patients has led to increased numbers of IAH and ACS being documented [1]. Raeburn and coworkers [2], for example, reported an incidence of 36% of ACS in trauma patients after damage control laparotomy. A considerable number of reports have demonstrated that IAH exerts deleterious effects on the cardiovascular system and on pulmonary, renal, and gastrointestinal function [3, 4, 5, 7]. Almost 30% of trauma patients requiring damage control laparotomy who show ACS develop multiple organ failure. Of interest, overall mortality associated with ACS is 43% [2]. This is in marked contrast to non-ACS trauma patients, with an incidence of multiple organ failure of only 12% and mortality of only 8% [2]. IAP monitoring was introduced because early intervention may prevent the deleterious effects of ACS.
Several techniques have been reported, including pressure measurements in the urinary bladder, stomach, and rectum [8]. All techniques, however, are associated with distinct disadvantages and limitations [8, 9], requiring the search of novel approaches, which may allow standardized and continuous monitoring of IAP [8, 10, 11, 12]. We investigated the interaction and relationship of the compartment pressure of the rectus sheath (CPRS) and IAH in a rodent model and demonstrate that continuous measurement of CPRS is a valid and simple approach for monitoring of IAH.
Materials and methods
Experiments were performed in accordance with German laws on the protection of animals and the NIH Guide for the Care and Use of Laboratory Animals. Six Sprague-Daley rats of either sex (367 ± 25 g body weight) were used. Rats were kept at a room temperature of 22 °C on 12-h light/dark cycles with free access water and standard laboratory chow.
Anesthesia and surgical preparation
The animals were anesthetized by isoflurane (Forene; Abbott, Wiesbaden, Germany). No curarization was administered. After oral intubation the animals were mechanically ventilated (7025-Rodent-Ventilator; Ugo-Basile, Comerio-Varese, Italy) with a tidal volume of 1 ml/100 g body weight. The fraction of inspired oxygen was 1.0 to provide optimal oxygenation. The positive end-expiratory pressure was set at 1 cmH2O. A baseline respiratory rate of 60 strokes/min was chosen to maintain normoventilation. The animals were placed in supine position. After laparotomy an 18-G catheter (Delta Ven-2; Delta Med, Viadana, Italy) was placed percutaneously into the peritoneal cavity and fixed with a purse-string suture to seal the abdominal wall cavity. A second 18-G catheter was inserted underneath the fascia into the rectus sheath and was also fixed with a purse-string suture. The catheters were separated by the peritoneum, preperitoneal fat, posterior layer of the fascia, and rectus abdominis muscle and therefore positioned in two different anatomical compartments so that the proximity of the two catheters would not influence the readings. The catheters were connected to pressure transducers (Statham P23dB; Statham Instruments, Oxnard, USA) via stopcocks and the pressures displayed on a monitor (Servomed; Hellige, Freiburg, Germany). Then the peritoneal cavity was closed with a 4–0 running suture, including the peritoneal sheet and the fascia of the rectus muscle.
Induction of intra-abdominal hypertension
A simple closed system for IAH induction and adjustment was developed. A standard 1000 ml gelatin solution (Gelafundin 4%; Braun, Melsungen, Germany) was attached to the stopcock of the intraperitoneally placed catheter. Both the IAP and CPRS catheters were zeroed at the midaxillary line. The height of the infusion bag was adjusted according to the pressure readings to provide the individually chosen pressures. The stopcock was then opened to the intraperitoneal catheter to fill the peritoneal cavity and was kept open throughout the experiment to ensure a constant IAP even in case of peritoneal fluid resorption. IAP increase was achieved by stepwise elevating the height of the bag. To decompress the abdomen the infusion bag was put to distinct levels below the animal, allowing the intraperitoneal fluid to drain into the bag by gravidity. A close-up photograph of a rat with the IAP and CPRS catheters in place and a drawing of the technical set up are provided in S.F1 and S.F2 (see Electronic Supplementary Material).
Experimental protocol
The physiological IAP was measured before fluid instillation. The pressure was then increased stepwise (2 mmHg) to 40 mmHg by instillation of warmed (38 °C) gelatin solution. The corresponding CPRS measurements were recorded. The IAP was then reduced in steps of 2 mmHg until the initial IAP values were reached, and the corresponding pressures of the rectus sheath were again recorded. At each IAP level one CPRS measurement was performed. Measurements were first performed without closure of the skin of the laparotomy. Then, the skin was also closed with a 4–0 running suture, and the measurements were repeated.
Statistical analysis
Data are expressed as median ± range. After ensuring normality and equal variance in the distribution of values the physiological pressures were compared using the paired t test. The level of p < 0.05 was considered statistically significant. Bland-Altman analysis was performed to evaluate bias and agreement between CPRS and IAP [13, 14]. Stability of differences between IAP and CPRS over the entire range of IAP values and between animals was analyzed using repeated-measures analysis of variance. Variance components were estimated using restricted maximum likelihood.
Results
Median IAP before fluid instillation was 2 mmHg (range 2–4) and median CPRS was 6 mmHg (4–7). Comparison between physiological IAP and physiological CPRS demonstrated a statistically significant difference (p < 0.001). For IAP lower than 12 mmHg four or five measurements per animal and experimental setting were recorded according to the initial physiological IAP. Table 1 shows a Bland-Altman analysis comparing IAP and CPRS in this low pressure range. Stepwise elevation in IAP resulted in a stepwise increase in CPRS with a very good agreement for IAP from 12 to 40 mmHg (Fig. 1a). In addition, the stepwise decompression of the IAH was associated with a stepwise decrease in CPRS with similar agreement between IAP and CPRS (Fig. 1b). Closure of the skin did not affect differences between IAP and CPRS (Fig. 1c, d). Variance components between steps of IAP within animals and those between animals were good for all experimental settings, confirming the small bias of the indirect measurements for IAP at or above 12 mmHg (Table 2). Moreover, correlations of the measurements of differences within animals were also weak (0.05–0.20).
Discussion
Herein we demonstrate for the first time that CPRS is strongly correlated with IAP, and that this correlation is independent of the direction of pressure changes and skin suture. In general, the abdominal wall is not considered a part of the abdominal compartment but may be influenced by IAH. This view is based on experiments [15] demonstrating a significant reduction in rectus sheath blood flow upon increased IAP. IAP elevations to 10 and 30 mmHg are associated with a reduction in rectus sheath blood flow to 58% and 24% of baseline values [15]. Our present results indicate that in IAH the abdominal wall must be considered as a part of the abdominal compartment with intramuscular pressures virtually identical to IAPs, although under physiological conditions IAP was significantly lower than CPRS. In our experiments the animals were not paralyzed. However, as indicated by our data, possible contractions of the rectus muscle clearly did not increase CPRS compared to IAP at levels of 12 mmHg or higher.
Of interest, skin closure did not affect CPRS measurement in our rodent model. However, we are aware that the extensibility of rodent skin may differ from that of humans, and therefore this result may not necessarily be transferred to the human setting but may need confirmation in a porcine experimental setup or human pilot studies. As recommended by the World Society on Abdominal Compartment Syndrome consensus definitions (http://www.wsacs.org/), a new method should be compared against a gold standard. We validated our technique against direct IAP measurement, which must be considered the most accurate and straight forward way to determine IAP [16]. Although it would have been of interest to compare CPRS against intravesical pressure measurement (IVP), IVP was not used for validation because there is little information on adequate volume priming of the bladder in the rat model.
Our finding has potential clinical implications. Monitoring of CPRS may be an alternative to other established indirect techniques for assessment of IAP. IVP is currently thought to be the gold standard for IAH monitoring, and various modifications have been proposed [8, 17, 18, 19]. However, although IVP shows a good correlation to IAP in most patients, there are distinct limitations to its application. IVP measurement in the presence of bladder trauma, peritoneal adhesions, pelvic fractures and hematoma, abdominal packing, or neurological bladder disorders may lead to overestimation of IAP [8]. In addition, the validity of measurements may depend on fluid instillation into the bladder before measurement [20, 21].
As with most other techniques currently in use, measurement of CPRS is an indirect method for assessing IAP. The technique may bear potential disadvantages such as local infections and intramuscular bleeding, which must be elucidated in further studies using large animal models. As with other fluid-filled systems for transmitting pressure, the pressure signal can be dampened by an air bubble and underestimate IAP, or the catheter can be blocked by a blood cloth and overestimate IAP. However, CPRS measurement may provide distinct advantages over other indirect techniques. It does not interfere with gastric contents, feeding tubes, pelvic packing, or bladder drainage. Following laparotomy for damage control or decompression CPRS may conveniently and safely be measured before closure of the subcutaneous layer and the skin. Percutaneous probe insertion under ultrasonic control may be another option. In special situations temporary CPRS measurement may also be used to validate the accuracy of other IAP measurement techniques, such as IVP in the presence of perivesicular hematoma, or to detect concomitant compartment syndrome of the rectus sheath after decompressive laparotomy.
The feasibility and value of repeated or continuous CPRS measurements for prolonged periods of time have not yet been evaluated. Thus the interaction and relationship of abdominal wall and peritoneal cavity pressures need further elucidation to delineate potential indications of CPRS measurements for monitoring of IAH in patients.
References
Rotondo MF, Schwab CW, McGonigal MD, Phillips GR 3rd, Fruchterman TM, Kauder DR, Latenser BA, Angood PA (1993) “Damage control”: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma 35:375–382
Raeburn CD, Moore EE, Biffl WL, Johnson JL, Meldrum DR, Offner PJ, Francoise RJ, Burch JM (2001) The abdominal compartment syndrome is a morbid complication of postinjury damage control surgery. Am J Surg 182:542–546
Diebel LN, Dulchavsky SA, Brown WJ (1997) Splanchnic ischaemia and bacterial translocation in the abdominal compartment syndrome. J Trauma 43:852–855
Balogh Z, McKinley BA, Holcomb JB, Miller CC, Cocanour CS, Kozar RA, Valdivia A, Ware DN, Moore FA (2003) Both primary and secondary abdominal compartment syndrome can be predicted early and are harbringers of multiple organ failure. J Trauma 54:848–859
Sugrue M, Jones F, Lee A, Buist MD, Deane S, Bauman A, Hillman K (1996) Intraabdominal pressure and gastric intramucosal pH: Is there an association? World J Surg 20:988–991
Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, Japiassu A, Kurtop E, De Keulenaer BL, Del Turco M, Cosimini P, Ranieri M, Jacquet L, Laterre PF, Gattinoni L (2004) Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med 30:822–829
Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri M, Del Turco M, Wilmer A, Brienza N, Malcangi V, Cohen J, Japiassu A, De Keulenaer BL, Daelemans R, Jacquet L, Laterre PF, Gunther F, de Souza P, Cesana B, Gattinoni L (2005) Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med 33:315–322
Malbrain MLNG (2004) Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med 30:357–371
Sugrue M (2002) Intra-abdominal pressure: time for clinical practice guidelines? Intensive Care Med 28:389–391
Schachtrupp A, Tons C, Fackeldey V, Hoer J, Reinges M, Schumpelick V (2003) Evaluation of two novel methods for the direct and continuous measurement of the intra-abdominal pressure in a porcine model. Intensive Care Med 29:1605–1608
Balogh Z, Felicity Jones, D'Amours S, Parr M, Sugrue M (2004) Continuous intra-abdominal pressure measurement technique. Am J Surg 188:679–684
Deeren DH, Dits H, Malbrain MLNG (2005) Correlation between intra-abdominal and intracranial pressure in nontraumatic brain injury. Intensive Care Med 31:1577–1581
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurements. Lancet I:307–310
Bland JM, Altman DG (1995) Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 346:1085–1087
Diebel L, Saxe J, Dulchavsky S (1992) Effect of intra-abdominal pressure on abdominal wall blood flow. Am Surg 58:773–775
De Potter TJ, Dits H, Malbrain ML (2005) Intra- and interobserver variability during in vitro validation of two novel methods for intra-abdominal pressure. Intensive Care Med 31:747–751
Cheatham ML, Safcsak K (1998) Intraabdominal pressure: a revised method for measurement. J Am Coll Surg 186:594–595
Iberti TJ, Kelly KM, Gentil DR, Hirsch S, Benjamin E (1987) A simple technique to accurately determine intraabdominal pressure. Crit Care Med 15:1140–1142
Sugrue M (1995) Intra-abdominal pressure. Clin Intensive Care 6:76–79
Fusco MA, Shayn MR, Chang MC (2001) Estimation of intra-abdominal pressure by bladder pressure measurement: validity and methodology. J Trauma 50:297–302
Gudmundsson FF, Viste A, Gislason H, Svanes K (2002) Comparison of different methods for measuring intra-abdominal pressure. Intensive Care Med 28:509–514
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Meier, C., Schramm, R., Holstein, J.H. et al. Measurement of compartment pressure of the rectus sheath during intra-abdominal hypertension in rats. Intensive Care Med 32, 1644–1648 (2006). https://doi.org/10.1007/s00134-006-0366-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0366-4