Abstract
Objectives
To evaluate the effects of arm exercise with or without support of mechanical ventilation on breathing pattern, respiratory muscle pressure output, and ratings of dyspnea and arm discomfort in difficult-to-wean patients with COPD.
Design and setting
Prospective, controlled, physiological study in a respiratory ICU.
Patients
Eight tracheostomized difficult-to-wean patients.
Intervention
Patients performed an incremental and an endurance arm exercise while breathing through a trach collar or while receiving pressure support ventilation.
Measurements and results
Breathing pattern, mechanics, arterial saturation, heart rate, and subjective ratings of dyspnea and arm discomfort were measured at baseline, at the end, and 30 min after exercise. Exercise during pressure support ventilation was found to result in higher peak workload (incremental testing) than exercise during trach collar. Moreover, compared to incremental and endurance testing during trach collar, incremental and endurance testing during pressure support ventilation resulted in greater tidal volume, and lower respiratory rate, lower pressure output from the respiratory muscles, and lower work of breathing. Exercise-induced worsening of dyspnea and arm discomfort during trach collar was similar to the corresponding values recorded during pressure support ventilation.
Conclusion
In tracheostomized difficult-to-wean patients with COPD arm exercise performed during unassisted respiration (trach collar) causes greater increases in respiratory rate and in respiratory muscle pressure output than arm exercise performed during pressure support ventilation. Exercise-induced dyspnea and arm discomfort are similar during assisted and nonassisted respiration.
Similar content being viewed by others
Introduction
Several studies have investigated mechanisms that contribute to exertional dyspnea and reduced exercise capacity in patients with chronic obstructive pulmonary disease (COPD); these include severe peripheral muscle wasting [1, 2, 3], increased intrinsic mechanical loading of inspiratory muscles and onset of dynamic hyperinflation [3, 4, 5], increased mechanical restriction of the thorax [6], inspiratory muscle weakness [3, 6, 7], gas exchanges abnormalities [6], and any combination of the above [6]. Numerous strategies have been proposed and tested for enhancing exercise performance, including noninvasive ventilation [8, 9, 10]. In outpatients with COPD strategies aimed at enhancing physical performance have focused on exercise training of lower [6] and upper limbs [11, 12, 13]. In patients with COPD, supported and unsupported arm exercise capacity is decreased, and arm exercise can trigger severe dyspnea [14, 15, 16]. This is important since arm activity is involved in the performance of most activities of daily living. Dynamic hyperinflation during arm exercise in patients with COPD [17] may contribute to dyspnea with reduced exercise capacity.
Since even patients with forced expiratory volume in 1 s below 40% of the predicted and patients with hypercapnia (Gold stage IV) may tolerate high-intensity training stimuli [7], they should be offered exercise training [10]. Moreover, as many as 30% of patients successfully weaned from mechanical ventilation for 2 days tolerate early institution of arm exercise training in addition to general physiotherapy [7]. In these patients weaned from mechanical ventilation [7] the addition of daily upper limb training to general physiotherapy enhances exercise tolerance and reduces both dyspnea and arm discomfort during exercise. More detailed information is needed on physiological variations during training in very severe COPD patients admitted to ICU and whether different training programs (focusing on resistance or endurance) exert different subjective and physiological changes.
The aim of this study was to evaluate the effects of arm exercise with or without support of mechanical ventilation on breathing pattern, respiratory mechanics, respiratory muscle pressure output, and ratings of dyspnea and arm discomfort in difficult-to-wean patients with COPD. Some of the results of the present study have been previously reported in the form of an abstract [18].
Methods
Patients
The study included eight tracheostomized difficult-to-wean patients with COPD who had been mechanically ventilated for at least 15 days, and who were admitted to our unit between 31 October 2003 and 30 June 2004. All patients met criteria for the diagnosis of COPD according to the American Thoracic Society Criteria [19]. In our respiratory intensive care unit (RICU) the patients underwent usual weaning protocols according to previously described methods [20]. Table 1 summarizes their anthropometric and clinical characteristics, maximal inspiratory pressure, and arterial blood gases. Seven of the eight patients had been treated with intravenous corticosteroids. During their stay in the RICU five patients followed a protocol of decreasing levels of pressure support and three underwent increasing periods of spontaneous breathing. At the time of the study entry all patients were mechanically ventilated on pressure support (13 ± 3 cmH2O) with a mean positive end-expiratory pressure set at 3.8 ± 1.1 cmH2O (Evita 2 Drager, Moislinger, Germany). Patients undergoing increasing periods of spontaneous breathing during trach collar spent 4 ± 2 h/day in that condition. Patients with concomitant neurological diseases, cancer, or other severe systemic diseases were excluded from the study. Likewise patients with hemodynamic instability, lung or systemic infections, myopathy, cardiovascular instability, orthopedic problems, insufficient cooperative state or any other condition involving inability to perform arm-ergometry and/or to maintain the sitting upright position were also excluded from the study. The study was approved by the Ethics Committee of The Salvatore Maugeri Foundation, IRCCS Gussago. Informed consent was obtained from all patients before enrollment into the study.
Measurements
The following data were recorded: (a) Anthropometrics and clinical conditions: age, body mass index, use of systemic corticosteroids at the study time, causes of relapse of COPD before admission in the ICU, RICU length of stay before admission, Acute Physiology and Chronic Health Evaluation (APACHE) II score at RICU admission, days of tracheotomy. (b) Maximal inspiratory pressure was measured through a cannula with cuff inflated and directly through the stoma with the inner balloon cuffed and without any inner tube. (c) Arterial blood gases were measured by means of an automated analyzer (RapidLab 865, Bayer, East Walpole, Mass., USA) on blood samples from the radial artery with patients under assisted ventilation at an FIO2 able to maintain SpO2 above 92%. (d) Noninvasive blood pressure measure was automatically monitored (Propac; Drägerwerk, Lübeck, Germany) at baseline, every 2 min of exercise, at the end of exercise, and during the recovery time. (e) Breathing pattern and mechanics (tidal volume, respiratory rate, pressure-time product, pressure-time index, dynamic intrinsic positive end-expiratory pressure, and respiratory drive) were obtained from flow and proximal airway pressure by means of a pneumotachograph/pressure transducer (Bicore, Irvine, Calif., USA) inserted at the end of the tracheostomy cannula and by means of an esophageal catether (Bicore) as previously described [21]. Dynamic intrinsic positive end-expiratory pressure was measured as the negative deflection in esophageal pressure (Pes) from the onset of inspiratory effort to the onset of inspiratory flow [21]. Respiratory drive was calculated as the change in Pes occurring between the time 100 ms prior to the start of airflow and the onset of flow [21]. The pressure-time product of the inspiratory muscles was measured as the area under the curve of Pes [21]. Pressure-time product was multiplied by respiratory rate and expressed in cmH2O per minute. The pressure-time index was calculated as Pes/Pesmax × (inspiratory time/total cycle duration). (f) An average of 180 s recording during spontaneous breathing was used for baseline and recovery times. To respect the times required by the experimental setting recordings at iso-workload, isotime, and end of incremental and endurance tests were limited to 10 s. The data used for statistical analysis at baseline, during exercise, and at recovery time were the average of at least five breaths free of artifacts. (g) Arterial oxygen saturation and pulse rate were monitored continuously by means of a pulse oximeter (Propac; Drägerwerk, Lübeck, Germany) during the study period. Data recorded for statistical analysis were an average value of the last 10 s immediately before the beginning of exercise (baseline), of 10 s immediately after the end of the exercise (end exercise) and of the last 10 s of the recovery time. (h) The need of oxygen supplementation during the study was also recorded. Oxygen supply was provided to all patients while mechanically ventilated to maintain SpO2 above 92%. (i) a symptom-limited incremental arm exercise test was performed on an isotonic arm ergometer (Monark 881, Stockholm, Sweden) secured to a table at the patient's shoulder level using the standard [7] 1-min incremental exercise protocol (Fig. 1). After stabilization and following a 1-min period of unloaded cycling at 40–45 cycles per minute the load was increased by 2.5 W/min. The patients were strongly encouraged to exercise to the point of intolerable breathlessness, discomfort or exhaustion, until the maximal heart rate was achieved or an abnormal electrocardiogram was noted or whenever the patient asked for stopping. The endurance arm exercise test was performed on the same arm ergometer at 50% of the peak workload reached during the incremental test. The endurance test was always performed the day after the incremental test. Patients were instructed to maintain the assigned work rate until exhaustion.
Study protocol
All eligible patients were assessed at baseline. After obtaining inform consent 200 μg salbutamol was administered through the tracheotomy tube with a spacer. Twenty minutes later patients were suctioned and after 10 min instructed to perform the arm-ergometer on two following days (incremental and endurance) first while on trach collar and then while on pressure support ventilation. During exercise testing on pressure support the level of ventilator assistance was the same as during resting conditions. The baseline incremental and endurance tests were performed by a physician not involved in the weaning process. Breathing pattern, respiratory mechanics, SpO2 and pulse rate were continuously monitored 30 min before, during, and until 30 min after the exercise tests. Perceived dyspnea and arm discomfort were quantified with a modified Borg [22] scale immediately before the start (t0), at the end (t1), and 30 min after the end of the tests (t2). Reasons for stopping the study included at least two of the following symptoms/signs: severe general discomfort with poor exercise tolerance, abdominal paradox, severe desaturation (SpO2 < 85%) despite 8 l/min oxygen or FIO2 greater than 0.4, arrhythmia, systolic blood pressure higher than 200 or lower than 70 mmHg at three consecutive measurements. All measurements were performed and recorded under the supervision of a respiratory therapist not involved in the study.
Statistical analysis
Results are shown as mean ± SD. Two-way analysis of variance for repeated measures was performed to assess the differences in physiological continuous variables between conditions (spontaneous breathing vs. inspiratory pressure support) and within conditions (baseline, isowatt for incremental test and at isotime for endurance test and 30 min after the end of the test) under tests. Friedman's test was used to compare repeated measures of perceived dyspnea and arm discomfort in either condition. The paired t test was employed to assess differences between means when appropriate. A p value less than 0.05 or less was considered statistically significant. Computations were performed using SPSS 12.0 package (SPSS, Chicago Ill., USA).
Results
Incremental test
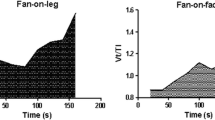
Patients achieved a greater peak workload (10 ± 6 vs. 7 ± 5 W, p < 0.02) while exercising on pressure support ventilation than while exercising on tracheotomy collar (Table 2). To maintain saturation above 85% FIO2 was increased in two patients during the incremental test both while on trach collar and while on pressure support. Throughout the exercise session performed while delivering pressure support ventilation, the tidal volume was greater (p < 0.0001) whereas respiratory rate (p < 0.001), pressure-time index (p < 0.004) and pressure-time product (p < 0.011) were lower than during exercise performed while on trach collar (Fig. 2). At comparable levels of effort (peak exercise for trach collar and isowatts or isotime for pressure support), rapid shallow breathing (defined as respiratory rate/tidal volume greater than 105 breaths × min–1 × ml–1) was not observed during incremental test or endurance test sustained either during pressure support or trach collar respiration.
Endurance test
Results of the endurance test while on trach collar were 3.53 ± 3.25 min and while on pressure support 4.78 ± 3.68 min (Table 3). Compared to exercise while on trach collar, exercise on pressure support was associated with greater tidal volume (p < 0.001), lower respiratory rate (p < 0.001), pressure-time index (p < 0.003), and lower pressure-time product (p < 0.005). Respiratory drive and dynamic hyperinflation at isowatt and isotime, albeit not significantly (Tables 2, 3) were less when endurance testing was performed during pressure support than during trach collar. In comparison to baseline, all patients showed a statistically poorer dyspnea sensation at peak time under both unassisted and assisted breathing (p < 0.001) and arm discomfort (p < 0.002) Worsening of dyspnea and arm discomfort during endurance exercise were the same during trach collar and during pressure support ventilation (Fig. 3).
Discussion
The results of this study show that in tracheostomized difficult-to-wean patients with COPD arm exercise performed during unassisted respiration (trach collar) causes greater increases in respiratory rate and in respiratory muscle pressure output than arm exercise performed during pressure support ventilation. Exercise-induced dyspnea and arm discomfort are similar during assisted and nonassisted breathing.
Breathlessness is the most common symptom limiting exercise in patients with chronic obstructive pulmonary disease (COPD) [8]. In the latest stages of the disease even simple activities of daily living cannot be accomplished without dyspnea. In a previous study conducted in difficult-to-wean tracheostomized patients with COPD, meal time increased respiratory rate, end-tidal CO2, and dyspnea [23]. Pressure support ventilation prevented meal-time associated dyspnea [23].
Greenleaf et al. [24] and Nava et al. [25] reported that in patients admitted to ICUs and undergoing prolonged mechanical ventilation early exercise training aimed at functional recovery of different muscle groups is effective in reverting deconditioning and muscle wasting. More recently we reported [7] that in patients recently weaned from mechanical ventilation the addition of upper-extremity training to general physiotherapy exerts larger improvements both in exercise tolerance and in perceived dyspnea and muscular discomfort. For these reasons we hypothesized that an earlier arm training in COPD patients during the weaning period would be feasible provided that they were protected by mechanical ventilation. To our knowledge, there are no studies assessing changes in respiratory mechanics during sustained upper limb exercise, nor the hypothetical protective effect of mechanical ventilation.
In the first stages of spontaneous breathing (trach collar) our patients showed increased respiratory effort (pressure-time index) and increased respiratory muscle pressure output (pressure-time product), the latter being an indirect index of respiratory muscle oxygen consumption. Although the peak workload attained was (as a mean) as low as 8 W, at the end of the unassisted exercise all patients showed worsened respiratory muscle indices with increased respiratory drive and intrinsic PEEP. While on trach collar, our patients achieved a mean workloads of 7.2 ± 4.5 W; this is not surprising considering that our patients were in the process of being weaned from mechanical ventilation while in the Porta et al. [7] study (10 ± 3 W) all patients had already been successfully weaned for 48 h.
The results of the present study confirm those of previous studies in which noninvasive mechanical ventilation [5, 26, 27] resulted in an unloading of respiratory muscles and in a reduction in intrinsic positive end-expiratory pressure. These effects (among other mechanisms) may be considered the main reasons of the improvement in exercise tolerance with less perceived dyspnea. Dynamic intrinsic positive end-expiratory pressure and respiratory drive increased along with the increasing of exercise workload (Table 2). The reduction in minute ventilation at identical work rate and/or time observed during assisted ventilation due to lower tidal volume and respiratory rate is likely responsible for reduction in dynamic hyperinflation [28] and ultimately for improvement in exercise performance.
A recovery time of 30 min was sufficient to restore to the baseline mechanical condition; this relative short amount of time is probably due to the low workload attained during exercise. A constant work-rate exercise (endurance testing) was included in our protocol because we reasoned that endurance testing would better resemble activities of daily living. This in turn could facilitate assessment of the physiological responses to the adjunct of pressure support ventilation [9]. Physiological responses during constant work exercise during trach collar and during pressure support were similar to those observed during incremental test (Table 3).
In a meta analysis Van't Hul [9] reported that noninvasive ventilation reduces exertional dyspnea, while in another study [23] we demonstrated that the application of mechanical ventilation in tracheostomized patients during meals prevents worsening of dyspnea. In contrast to the former investigations, the present study observed no protective effect of pressure support ventilation on dyspnea during upper-limb exercise. Nevertheless, subjective ratings of dyspnea were lower when exercise was assisted than with spontaneous breathing both at iso-workload and at peak exercise. The individual data of perceived dyspnea and peripheral muscle discomfort both during incremental (Fig. 3) and endurance (Table 3) tests clearly show a common trend among patients and that mechanical ventilation reduces the between-subjects variability of data. Similarly to what observed for respiratory mechanics, 30 min of recovery was sufficient to restore to the baseline condition of perceived symptoms.
Limitations of the present study are the small sample size and the absence of a gastric balloon to measure the transdiaphragmatic pressure; furthermore, an enhanced exercise tolerance does not necessarily translate into an increased activity of daily living. Although several position papers have pointed out the importance of specific rehabilitation programs in the intensive care setting [29, 30, 31], the role of physiotherapy in ICU is still debated. Feasibility and effectiveness of upper limb training had been previously demonstrated in the intensive care setting in patients recently weaned from mechanical ventilation [7]. The results of our study suggest that arm training might be feasible in selected patients even during the period of active weaning.
In conclusion this study is the first to show that in tracheotomized difficult-to-wean patients with COPD arm exercise performed during unassisted respiration (trach collar) causes greater increases in respiratory rate and in respiratory muscle pressure output than arm exercise performed during pressure support ventilation. Exercise-induced dyspnea and arm discomfort are similar during assisted and nonassisted respiration.
References
American Thoracic Society/European Respiratory Society (1999) Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159:S1--S40
Debigare R, Cote CH, Maltais F (2001) Peripheral muscle wasting in chronic obstructive pulmonary disease. Clinical relevance and mechanisms. Am J Respir Crit Care Med 164:1712--1717
Laghi F, Tobin MJ (2003) Disorders of the respiratory muscles. Am J Respir Crit Care Med 168:10--48
Rossi A, Polese G, Brandi G, Conti G (1995) Intrinsic positive end-expiratory pressure (PEEPi). Intensive Care Med 21:522--536
O'Donnell DE, D'Arsigny C, Webb KA (2001) Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163:892--898
O'Donnell DE (1998) Exertional breathlessness in chronic respiratory disease. In: Mahler DA (ed) Dyspnea. Dekker, New York, pp 97--147
Porta R, Vitacca M, Gilè LS. Clini E, Bianchi L, Zanotti E, Barbano L, Ambrosino N (2005) Supported arm training in patients recently weaned from mechanical ventilation. Chest 128:2511--2520
Ambrosino N, Strambi S (2004) New strategies to improve exercise tolerance in chronic obstructive pulmonary disease. Eur Respir J 24:313--322
Van t Hul A, Kwakkel G, Gosselink R (2002) The acute effects of noninvasive ventilatory support during exercise on exercise endurance and dyspnea in patients with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil 22:290--297
Troosters T, Casaburi R, Gosselink R, Decramer M (2005) Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 172:19--38
Martinez FJ, Vogel PD, Dupont DN, Stanopoulos I, Gray A, Beamis JF (1993) Supported arm exercise vs unsupported arm exercise in the rehabilitation of patients with severe chronic airflow obstruction. Chest 103:1397--1402
Epstein SK, Celli BR, Williams J, Tarpy S, Roa J, Shannon T (1995) Ventilatory response to arm elevation. Its determinants and use in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 152:211--216
Epstein SK, Celli BR, Martinez FJ, Couser JI, Roa J, Pollock M, Benditt JO (1997) Arm training reduces the V'O2 and V'E cost of unsupported arm exercise and elevation in chronic obstructive pulmonary disease. J Cardiopulm Rehabil 17:171--177
Celli BR, Rassulo J, Make BJ (1986) Dyssynchronous breathing during arm but not leg exercise in patients with chronic airflow obstruction. N Engl J Med 314:1485--1490
Criner GS, Celli BR (1988) Effect of unsupported arm exercise on ventilatory muscle recruitment in patients with severe chronic airflow obstruction. Am Rev Respir Dis 138:856--861
McKeough ZJ, Alison JA, Bye PT (2003) Arm exercise capacity and dyspnea ratings in subjects with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 23:218--225
Gigliotti F, Coli C, Bianchi R, Grazzini M, Stendardi L, Castellani C, Scano G (2005) Arm exercise and hyperinflation in patients with COPD: effect of arm training. Chest 128:1225--1232
Vitacca M, Bianchi L, Sarvà M, Paneroni M, Balbi B (2005) Physiological variations during supported arm-training in tracheostomized patients. Eur Respir J 26 [Suppl 49]:A3422, 527s
American Thoracic Society (1995) Standards of the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 152 [Suppl]:S77--S120
Vitacca M, Vianello A, Colombo D, Clini E, Porta R, Bianchi L, Arcaro G, Guffanti E, Lo Coco A, Ambrosino N (2001) Comparison of two methods for weaning COPD patients requiring mechanical ventilation for more than 15 days. Am J Respir Crit Care Med 164:225--230
Vitacca M, Porta R, Bianchi L, Clini E, Ambrosino N (1999) Differences in spontaneous breathing pattern and mechanics in patients with severe COPD recovering from acute exacerbation. Eur Respir J 13:365--370
Borg GAV (1992) Psychophysical basis of perceived exertion. Med Sci Sports Exerc 14:377--381
Vitacca M, Callegari G, Sarvà M, Bianchi L, Barbano L, Balbi B, Ambrosino N (2005) Physiological effects of meal in difficult-to wean tracheotomised patients with chronic obstructive pulmonary disease. Intensive Care Med 31:236--242
Greenleaf JE (1997) Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc 29:207--215
Nava S (1998) Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil 79:849--854
Maltais F, Reissmann H, Gottfried SB (1995) Pressure support reduces inspiratory effort and dyspnea during exercise in chronic airflow obstruction. Am J Respir Crit Care Med 151:1027--1033
Kyroussis D, Polkey MI, Keilty SE, Mills GH, Hamnegard CH, Moxham J, Green M (1996) Exhaustive exercise slows inspiratory muscle relaxation rate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 153:787--793
O'Donnell DE, Revill SM, Webb KA (2001) Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164:770--777
Stiller K (2000) Physiotherapy in intensive care. Towards an evidence-based practice. Chest 118:1801--1813
Fishman AP (1994) Pulmonary rehabilitation research: NIH workshop summary. Am Rev Respir Crit Care Med 149:825--833
Foster S, Thomas HM (1990) Pulmonary rehabilitation in lung disease other than chronic obstructive pulmonary disease. Am Rev Respir Dis 141:601--604
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vitacca, M., Bianchi, L., Sarvà, M. et al. Physiological responses to arm exercise in difficult to wean patients with chronic obstructive pulmonary disease. Intensive Care Med 32, 1159–1166 (2006). https://doi.org/10.1007/s00134-006-0216-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0216-4