Abstract
Objective
To evaluate the effect of positive end-expiratory pressure on the sound filtering characteristics of injured lungs.
Design and setting
Prospective experimental study in the animal laboratory in an academic medical center.
Patients and participants
Six 35- to 45-kg anesthetized, intubated pigs.
Interventions
Acute lung injury with intravenous oleic acid.
Measurements and results
We injected a multifrequency broad-band sound signal into the airway while recording transmitted sound at three locations bilaterally on the chest wall. Oleic acid injections effected a severe pulmonary edema predominantly in the dependent lung regions, with an average increase in venous admixture from 16±14% to 57±13% and a reduction in static respiratory system compliance from 31±6 to 16±3 ml/cmH2O. A significant concomitant increase in sound transfer function amplitude was seen in the dependent and lateral lung regions; little change occurred in the nondependent areas. The application of PEEP resulted in a decrease in venous admixture, increase in respiratory system compliance, and return of the sound transmission to preinjury levels.
Conclusions
Acute lung injury causes regional acoustic transmission abnormalities that are reversed during alveolar recruitment with PEEP.
Similar content being viewed by others
Introduction
The development of acute lung injury is characterized by loss of alveolar capillary membrane integrity, accumulation of fluid in the extravascular space, and loss of gas volume, particularly in the dependent parts of the lungs. The presence of areas of low ventilaton/perfusion ratios and frank atelectasis or consolidation lead to the clinical manifestations of respiratory failure: arterial hypoxemia and impaired lung mechanics. We have shown previously that pathophysiological changes during the development of experimental acute lung injury effect an increase in the transmission amplitude of sound introduced into the airway [1]. When measured at multiple sites around the chest, this increase in sound transmission corresponded to the distribution of lung pathology, being largest in the dependent lung regions where the most extensive lung injury was found on radiographs and at postmortem examination. Sound transmission through injured areas of the lung likely is enhanced by an increase in lung tissue density which improves the matching of acoustic impedance between the lung parenchyma and the chest wall. This results in less sound energy being reflected back at this interface, allowing more sound energy to penetrate the chest wall to the surface sensor.
Positive end-expiratory pressure (PEEP) or continuous positive airway pressure have been an integral part of the clinical management of acute lung injury for several decades [2]. The application of PEEP or continuous positive airway pressure allows reinflation of collapsed lung segments and improvement in the ventilation/perfusion relationships of areas with low ventilation/perfusion ratios. When collapsed lung is recruited, the acoustic impedance mismatch at the lung-chest wall interface is reestablished. This causes more sound to be reflected back and less transmitted to the surface. We hypothesized that as the gas volume of the injured lung is increased toward normal with positive pressure therapy, the abnormal sound conduction characteristics should revert toward normal as well, bearing some relationship with the improvement in gas exchange and respiratory system mechanics.
Methods and materials
After approval by the Institutional Animal Care and Use Committee six healthy pigs weighing 35–45 kg and cared for according to the current guidelines for the care and use of laboratory animals were included in the study. Details of our standard anesthetic management and instrumentation for this experimental preparation have been published previously [1]. The experimental setup is shown in Fig. 1. To measure sound transmission through the respiratory system broad-band noise (350–4000 Hz) was generated digitally and injected into the airways via a connector at the proximal end of the endotracheal tube. This signal was recorded simultaneously with one sensor placed at the point of its entry into the breathing circuit, and with six sensors positioned on the surface of the chest at the level of the diaphragmatic dome. The chest sensors were placed bilaterally over the dependent lung 5 cm laterally from the spine, on the nondependent lung 5 cm from the sternum, and on the lateral midlung half-way between the other two locations. The data acquisition and the determination of the transfer function magnitude, coherence, and phase for each input-output pathway occurred on-line (Trans19 Software, Technion, Haifa, Israel). All sound measurements were made in duplicate, the second recording immediately following the first. For details please see the Electronic Supplementary Material.
Experimental protocol
Thirty minutes after instrumentation baseline respiratory volume and pressure recordings, blood gas sampling, hemodynamic measurements using a pulmonary artery catheter, and sound data collection were performed at ambient airway pressure without interrupting mechanical ventilation. They were repeated after 15 min of ventilation with 10 cmH2O PEEP and again after 15 min at ambient airway pressure. Acute lung injury was then induced with oleic acid, as previously described [1]; data collection was repeated after 15 min of ventilation with 0, 5, 10, and 15 cmH2O PEEP and concluded with two measurements 15 min apart at ambient airway pressure. Thereafter the animal was killed, and a postmortem examination of the lungs was performed.
In one animal the sound measurements were repeated at the end of the study at PEEP of 10 and 0 cmH2O, in this order. Thereafter this animal was transported to the radiology suite and computed tomography images were recorded at 0 and 10 cmH2O of PEEP.
Data analysis
Venous admixture and static respiratory system compliance were calculated using standard formulae. The frequency distribution of the sound transfer function amplitude and coherence were plotted for each recording covering a frequency band from 0 to 5000 Hz. A single frequency range of interest was then selected leaving out the low and high ends of the spectrum where coherence was not consistently high (Fig. 2). Average and peak amplitude and coherence for this frequency range were calculated for each input-output pathway and averaged across the duplicate measurements. The effect of PEEP on uninjured lungs was assessed by comparing data obtained during PEEP to recordings immediately before and after its application. The baseline representing normal lungs for comparison with measurements obtained during lung injury was calculated by averaging the two measurements at ambient airway pressure prior to injury.
Frequency distributions of coherence and transfer function amplitude measured at the posterior aspect of the left lung in a pig before and after induction of lung injury with oleic acid. All transfer function amplitude measurements (continuous lines) are plotted; the coherence (broken line) corresponds to the transfer function with the highest peak amplitude. Arrow The frequency band selected for analysis
The results are presented as mean ±SD. The statistical significance of the observed changes was evaluated using Friedman’s nonparametric analysis of variance or Wilcoxon’s signed-rank test because deviation from normal distribution and inequality of variances could not be reliably ruled out [3]. The strength of association between changes in sound transmission amplitude and physiological variables reflecting lung injury was tested with linear and exponential regression analysis. Correlation coefficients were determined for each subject and sensor individually and averaged over the six animals. We also report overall correlations based on population averages for anterior, lateral, and posterior lung regions, averaging the corresponding regions of both lungs. Results were considered statistically significant if the probability of type α error was less than 5%.
Results
Physiological measurements
The application of 10 cmH2O PEEP to uninjured lungs caused expected decreases in blood pressure, cardiac output, and SvO2 (ESM, Table 1). Venous admixture also decreased significantly for the duration of ventilation with PEEP. Induction of lung injury was associated with statistically significant tachycardia, systemic hypotension, and pulmonary hypertension, while cardiac output remained unchanged (Table 1). A 3.5-fold increase in venous admixture required oxygen supplementation to prevent profound hypoxemia. The static respiratory system compliance decreased to 51% of its baseline value. The stepwise application of 5–15 cmH2O PEEP decreased cardiac output but improved the respiratory system compliance and on the average brought the venous admixture to its baseline level. These effects of PEEP were reversed upon its discontinuation.
Sound measurements
While the input signal recorded at the outlet of the loudspeaker box was similar to the original white noise, it was considerably altered by passage through the circuit connectors and the tracheal tube, as we have previously observed [1]. The signal recorded on the chest surface consisted of peaks and valleys, with a frequency band from 1200 to 3500 Hz containing, without exception, the highest amplitudes and the largest changes during developing lung injury (Fig. 2). In this frequency band the coherence was invariably high with the exception of the frequencies constituting a valley in the transfer function amplitude. Hence high amplitude readings always came from frequencies where coherence was good. The average and peak transfer function amplitudes of the entire study group are shown in Table 2 and Fig. 3, respectively. All peak transfer function amplitude measurements from one single subject over the entire course of the study are shown in ESM (Fig. 1). When 10 cmH2O PEEP was applied to noninjured lungs, several of the sound pathways displayed a small decrease in peak and average transfer function amplitude. This effect was consistently statistically significant only for the left anterior sensor (ESM, Table 2).
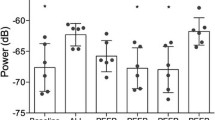
Peak amplitude of the lung sound transfer function relative to baseline level over a frequency band from 1200 to 3500 Hz, recorded with six sensors overlying three lung regions bilaterally, during development of oleic acid induced lung injury and subsequent application and withdrawal of PEEP. Data points are average values for all six animals. BL Baseline. For clarity, standard deviation bars are only shown for one region. Time-related changes are statistically significant for all regions
Oleic acid lung injury effected a statistically significant increase in the transfer function amplitude in all sensors except the left anterior one. These changes varied depending on sensor location, being on the average largest in the dependent and smallest in the nondependent parts of both lungs (Table 2, Fig. 3). The injury-induced increase in transfer function amplitude was statistically significantly larger in the right lung than the left (p<0.01).
Application of PEEP to injured lungs resulted in a statistically significant fall in the sound transmission amplitude of all sensors (Table 2, Fig. 3). Computed tomography in one animal showed severe volume loss in areas underlying the sensors exhibiting increased transmission (Fig. 4a), while aerated lung was seen under sensors with normal sound transmission. The application of 10 cmH2O PEEP in this subject resulted in almost complete reaeration of the lungs and marked reduction in the sound transmission abnormalities (Fig. 4b). Most of the decrease in transfer function amplitude occurred with 5 cmH2O PEEP, making the average amplitude no longer statistically significantly different from the preinjury value. Further decrease was seen at PEEP levels of 10 and 15 cmH2O; in fact the average transfer function amplitude was statistically significantly lower during 15 cmH2O PEEP than before lung injury (p<0.01). Withdrawal of PEEP resulted in a rapid increase in sound transmission so that 15 min after discontinuation of PEEP the amplitudes were no longer statistically significantly different from recordings made during lung injury without PEEP.
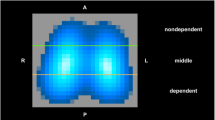
End-expiratory chest computed tomography images of a pig with oleic acid-induced lung injury ventilated with ambient airway pressure (a) and 10 cmH2O PEEP (b). The superimposed graphs display the frequency distributions of sound transfer function amplitude recorded from locations shown by the closest arrow. An abnormally increased sound transmission amplitude is recorded at locations overlying lung volume loss. These abnormalities disappear with PEEP-induced lung expansion
An exponential function gave the best fit for the relationship calculated over the phases of the study between the transfer function amplitude and physiological indicators of lung injury. In individual animals the coefficient of determination (R2) values for the six sensors ranged from 0.33±0.23 to 0.73±0.20 for the regression between transfer function amplitude and venous admixture, and from 0.35±0.38 to 0.45±0.18 for transfer function amplitude and static respiratory system compliance. Transfer function amplitudes averaged across both lungs at the nine phases of the study followed an exponential function in their association with venous admixture in the dependent and lateral lung fields, but not in the anterior, nondependent areas (Fig. 5).
Association between venous admixture and average peak sound transmission amplitude of the anterior (diamonds), lateral (squares), and posterior (dots) lung fields (solid dots) during development of oleic acid-induced lung injury and during lung inflation with PEEP. Data points are averages of all six animals at nine phases of the study
Postmortem examination of the lungs showed hemorrhagic pulmonary edema in the dependent one-third to one-half of both lungs. The nondependent parts of the lungs appeared well aerated. Between these two areas was a region of transition characterized by patchy discoloration. Asymmetry in the acoustic findings between the two lungs was always confirmed at postmortem examination as a corresponding difference in the extent of injury between the lungs.
Discussion
Recruitment of collapsed lung and maintenance of alveoli at an inflated state has evolved as one of the leading principles of modern respiratory support during acute lung injury. Unfortunately, the determination of when recruitment is successful still relies on indirect assessment based on global gas exchange and pulmonary mechanics. Localizing information is available by computed tomography [4], but since this requires transporting the patient to an imaging suite, it cannot be used effectively to direct respiratory therapy. Since an acoustic wavefront introduced into the airway is known to penetrate the respiratory system [5, 6], and since the propagation of sound is influenced by the composition of the medium in which it moves [7], it is feasible that the pathological changes that occur during lung injury might alter such a sound signal sufficiently to allow monitoring of its progression and regression. We have previously shown that analysis of an external sound signal as it is transmitted through the respiratory system can be used to detect and localize acute lung injury in experimental animals [1]. The results of the current study confirm these findings. They also indicate that as the gas volume of the injured lung is reestablished with positive airway pressure, that the sound transmission abnormalities disappear, and that withdrawal of positive airway pressure is quickly followed by their return.
We were careful to prevent any physical factors confounding our measurements during elevated airway pressure. Both the loudspeaker that produced the sound and the reference microphone that recorded the incident signal were subjected to airway pressure fluctuations during the mechanical breaths and during the application of PEEP. Pressurization of the circuit also changed the density of gas in the circuit and in the airways. To maintain the function of the loudspeaker during PEEP it was mounted in the airtight chamber in such a way that no pressure gradient developed across its cone when the breathing circuit pressure was elevated above ambient. Mahagnah et al. [8] have shown that the differences in density between air and 80/20 helium/oxygen mixture do not affect sound transmission in normal humans. Our own measurements using a test lung and a breathing circuit similar to the one used in the present study also show that the transfer function amplitude remains unchanged at circuit pressure levels from 0 to 20 cmH2O. Furthermore, an increase in gas density should facilitate sound transmission and thus does not explain attenuation of transmission seen with PEEP during lung injury. The loudspeaker enclosure was designed to prevent direct airborne transmission of sound from the source to the chest sensors. The fact that the frequency distribution of the transfer function amplitude showed the peak and valley pattern resulting from the effect of the endotracheal tube on the original signal is further proof that the sound recorded on the chest wall truly had passed through the respiratory system. Finally, the lack of a major PEEP-induced decrease in transfer function amplitude in normal lungs, and the fact that the transfer function amplitude increased only gradually after withdrawal of PEEP indicate that the observed changes in sound transmission truly resulted from the effects of PEEP on the lung and not on the signal or from a mechanical load on the sensors.
The characteristics of the injury in this study were comparable to results previously published by us and others [1, 9, 10]. Postmortem examination also confirmed a gravity-dependent inhomogeneity of the injury with severe volume loss in the dependent parts of the lungs and nearly normal-appearing lung in the nondependent areas. We have previously documented that the abnormalities of gas exchange and lung mechanics appear to be caused primarily by alveolar collapse, rather than fluid extravasation, because the lungs are fully expandable after removal and their wet-to-dry weight ratio increases relatively little [1]. This was evident in the present series as well because the abnormalities in gas exchange, pulmonary mechanics and sound transmission were highly sensitive and promptly responsive to PEEP. The lung injury was consistently associated with a large increase in the sound transfer function amplitude in the dependent and midlung regions. The magnitude of this change, five- to sevenfold compared to baseline in the dependent lung regions, was similar to that observed in our previous experiments [1].
The predominance of the injury in the right lung in several subjects was a finding that we had not previously observed. That this was a real phenomenon and not a result of gain imbalance between right and left sensors was demonstrated clearly by our observations at postmortem examination, which verified the asymmetry whenever the acoustic measurements suggested it. Data collected during this study do not allow us to speculate on the cause of this phenomenon with any certainty. Most importantly, a statistically significant increase in transfer function amplitude was seen in all sensors except the one overlying the left anterior lung field and all sensors that showed the injury pattern responded similarly to the changes in expiratory airway pressure.
The transfer function amplitude responded to stepwise application of PEEP with a stepwise decrease. On average the baseline transmission level was reached at PEEP of 10 cmH2O; a further increase in airway pressure tended to reduce sound transmission even below baseline. Notably, venous admixture and respiratory system compliance were still markedly abnormal at a PEEP of 10 cmH2O, the latter remained reduced even at a PEEP of 15 cmH2O. These results indicate that there is a certain degree or type of pathological change in the lung parenchyma that produces an increase in sound transfer function amplitude. Hence an amplitude equivalent to that of an uninjured lung does not mean that the lung parenchyma is normal, but merely that the mechanisms that cause higher sound transmission were removed or suppressed. The exponential relationship between venous admixture and compliance, on one hand, and transfer function amplitude, on the other (Fig. 5), also supports this finding; the application of mechanical ventilation and PEEP may reinflate the lung enough to decrease sound transmission to a near-baseline level without effecting an improvement of similar magnitude in gas exchange and lung mechanics. The computed tomography images that we obtained from one subject (Fig. 4) clearly show that there was loss of lung volume in areas underlying the sound sensors with increased transmission, and that the return of sound transmission to normal was associated with reinflation of the lung. However, the true nature of the pathology that increased sound transmission in this study cannot be determined without further study.
A small decrease in transfer function amplitude below baseline was seen both when PEEP was applied to uninjured lungs, and when 15 cmH2O of PEEP was applied to injured lungs. This finding can be explained in two ways: Firstly, it is possible, even likely, that the lungs in an anesthetized, mechanically ventilated animal always have an element of volume loss which, when corrected with PEEP, results in decreased sound transmission. Secondly, a reduction in lung density below normal with hyperinflation may induce a decrease in transfer function amplitude below that measured when the lung is at its normal volume. It is probable that in any given subject, both of these factors played a role depending on the location of the sensor.
Clinical studies over the past several decades have established the importance of maintaining alveolar recruitment without hyperinflation in ventilated patients with acute lung injury [2, 4, 11, 12, 13]. We have shown that as the application of PEEP improves the gas exchange and mechanics of an injured lung, the abnormal sound filtering characteristics induced by the injury return to their preinjury state. We do not yet know the range of abnormality within the spectrum of lung injury to which sound transmission is sensitive. However, since sound transmission can be measured safely, rapidly, and repeatedly at the bedside, further study of its utility in adjusting respiratory therapy in patients with acute lung injury is warranted.
References
Räsänen J, Gavriely N (2002) Detection of porcine oleic acid-induced acute lung injury using pulmonary acoustics. J Appl Physiol 93:51–57
Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, Lamy M, Marini JJ, Matthay MA, Pinsky MR, Spragg R, Suter PM (1998) The American-European Consensus Conference on ARDS. II. Ventilatory, pharmacologic, supportive therapy, study design strategies and issues related to recovery and remodeling. Intensive Care Med 24:378–398
Sachs L (1982) Applied statistics. Springer, Berlin Heidelberg New York
Gattinoni L, Pelosi P, Vitale G, Pesenti A, D’Andrea L, Mascheroni D (1991) Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure. Anesthesiology 74:15–23
Pasterkamp, H, Kraman SS, Wodicka GR (1997) Respiratory sounds. Advances beyond the stethoscope. Am J Respir Crit Care Med 156:974–987
Kraman SS, Bohadana AB (1989) Transmission to the chest of sound introduced at the mouth. J Appl Physiol 66:278–281
Donnerberg RL, Druzgalski CK, Hamlin RL, Davis GL, Campbell RM, Rice DA (1980) Sound transfer function of the congested canine lung. Br J Dis Chest 74:23–31
Mahagnah M, Gavriely N (1995) Gas density does not affect pulmonary acoustic transmission in normal men. J Appl Physiol 78:928–937
Neumann P, Hedenstierna G (2001) Ventilation-perfusion distributions in different porcine lung injury models. Acta Anaesthesiol Scand 45:78–86
Rosenthal C, Caronia C, Quinn C, Lugo N, Sagy M (1998) A comparison among animal models of acute lung injury. Crit Care Med 26:912–916
Pelosi P, D’Andrea L, Vitale G, Pesenti A, Gattinoni L (1994) Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med 149:8–13
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a CR20 grant from the Mayo Foundation
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Räsänen, J., Gavriely, N. Response of acoustic transmission to positive airway pressure therapy in experimental lung injury. Intensive Care Med 31, 1434–1441 (2005). https://doi.org/10.1007/s00134-005-2745-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2745-7