Abstract
Objective
We compared the effectiveness of a new continuous positive airway pressure (CPAP) device (neonatal helmet CPAP) with a conventional nasal CPAP system in preterm neonates needing continuous distending pressure.
Design and setting
Randomized, physiological, cross-over study in a tertiary referral, neonatal intensive care unit in a university teaching hospital.
Patients
Twenty very low birth weight infants with a postnatal age greater than 24 h who were receiving nasal CPAP for apnea and/or mild respiratory distress were enrolled.
Interventions
CPAP delivered by neonatal helmet CPAP and nasal CPAP in random order for two subsequent 90-min periods.
Measurements and results
Were continuously measured the Neonatal Infant Pain Scale (NIPS) score, oxygen requirements, respiratory rate, heart rate, oxygen saturation, transcutaneous PO2 (tcPO2) and PCO2 (tcPCO2), blood pressure, and desaturations. NIPS scores were significantly lower when the infants were on the neonatal helmet CPAP than when they were on nasal CPAP (0.26±0.07 vs. 0.63±0.12). The other studied parameters did not differ between the two CPAP modes. The number of deaturations was reduced during the neonatal helmet CPAP treatment (18 vs. 32), although this difference was not significant.
Conclusions
In this short-term physiological study the neonatal helmet CPAP appears to be as good as the golden standard for managing preterm infants needing continuous distending pressure, with enhanced tolerability. Further evaluation in a randomized clinical trial is needed to confirm these findings.
Similar content being viewed by others
Introduction
The use of continuous positive airway pressure (CPAP) in the neonatal intensive care unit (NICU) is widespread, especially in very premature infants. Several epidemiological studies have found that the avoidance of mechanical ventilation and the increased use of nasal CPAP for the treatment of respiratory distress syndrome (RDS) are associated with a decrease in bronchopulmonary dysplasia [1, 2] In addition, CPAP is effective in patients during the postextubation phase to prevent atelectasis and to reduce apnea episodes and the need of reintubation [3].
Since 1971 when Gregory et al. [4] originally described the use of a simple device to provide CPAP as a way to maintain lung gas volumes in preterm infants with RDS, newer and more specific devices have been developed. CPAP can now be delivered in preterm infants by means of nasal prongs or nasopharyngeal tubes, nasal masks or face masks, and nasal cannulae [5, 6]. These techniques seem to work effectively; however, they may fail due to increased work of breathing or discomfort of the patient. In particular, the reasons for the failure of nasal CPAP, the most used method, were recently reported as: “insufficiently applied pressure, insufficient circuit flow, inappropriate prong size or placement, airway obstruction from secretions, and a baby’s open mouth creating a large leak and lowering the pharyngeal pressure” [6]. In addition, nasal prongs often become dislodged, making care of these infants difficult. For example, severe nasal skin necrosis was reported as a complication of this therapy [7]. Improving the interface between patient and ventilator thus seems crucial to achieving a prolonged and effective application of CPAP in preterm infants.
To improve the patient-ventilator interface we developed a new device (neonatal helmet CPAP) to administer CPAP in preterm infants. In this study we tested it for the first time. We postulated that the neonatal helmet CPAP could have important advantages: (a) ease of use, (b) good tolerability, (c) reduced risk of disconnection from CPAP, (d) absence of air leakage due to baby’s open mouth, maintaining a stable pressure in the system, and (e) a fixation system to avoid the risk of cutaneous lesions. Some of these advantages have been in adults demonstrated using a similar device [8, 9]; however, the role of the helmet for noninvasive positive pressure ventilation has not yet been completely defined [9, 10, 11].
The purpose of this study was to evaluate the effectiveness of the neonatal helmet CPAP in terms of infant’s comfort as an alternative to conventional nasal CPAP to treat premature infants needing continuous distending pressure. We hypothesized that the neonatal helmet CPAP can maintain ventilation and oxygenation with greater tolerability of the patient than in conventional nasal CPAP.
Methods
Technical aspects
The neonatal helmet CPAP (Starmed, Mirandola, Modena, Italy) is made of rigid transparent polycarbonate. It consists of a bed with two basic parts. In the upper part of the bed there is a sealed hood, to which the inspiratory line of the circuit is directly connected by a dedicated port. At this level pressure, fraction of inspired oxygen (FIO2), and temperature in the chamber are detected and continuously displayed (Sensor O.P.T., Starmed). Another port is provided for expiratory exit in which an adjustable positive end-expiratory pressure valve allows the desired pressure to be regulated in the system. The sealed hood has a simple opening system to permit the prompt accessibility to the infant’s face if necessary. In addition, in the upper part of the hood there is a pressure release (pop-off) valve that prevents excessive pressure build-up in the system (10 cmH2O). The pressure chamber is kept separated from the rest of the bed by a transparent Latex-free polyurethane membrane. The cone-shaped membrane has a hole in the middle to allow the patient’s head to pass through. Due to the pressure in chamber the soft membrane becomes a loose collar around the neck, adhering to the shoulders of the patient with a sealing and atraumatic effect. A soft diaper system positioned in the lower part of the patient’s body allows proper sealing and shoulder contact of the patient to the soft membrane (Fig. 1).
The volume of the space around the head of the patient within the pressure chamber is approx. 2.5 l; however, the dead-space effect is negligible because of the high continuous flow of fresh gases delivered through the chamber (range 8–10 l/min). The neonatal helmet CPAP is available in two different sizes. The small size is for patients with birth weight less than 1500 g (diameter of the hole in the membrane 3 cm), and the large size is for neonates with birth weight 1500–2500 g (diameter of the hole in the membrane 4 cm).
As standard nasal CPAP system we used the Infant Flow Device (IFD; Hamilton Medical, Reno, Nev., USA; manufactured by Electro Medical Equipment, Brighton, UK). The CPAP pressure was set by varying the flow rate, and the largest prongs that fit easily into the nostrils were used in each infant [12]. Both devices were calibrated against an independent oxygen analyzer to determine any differences in oxygen delivery.
Study design and patient selection
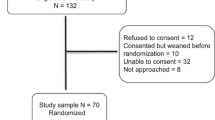
The study was conducted in the NICU at the Pediatric Department, Medical School, University of Padua between January 2003 and May 2003. Premature infants with a postnatal age greater than 24 h who were receiving nasal CPAP for apnea and/or mild respiratory distress and were otherwise medically stable were eligible for the enrollment. An interim analysis was performed after 20 patients were studied. The p value for Neonatal Infant Pain Scale (NIPS) was less than 0.005, and therefore the study was terminated, and a total of 20 infants were enrolled into this study. No infants were withdrawn during the study due to intolerance to CPAP or clinical deterioration. Descriptive characteristics of the 20 studied infants are summarized in Table 1.
The study used a cross-over design that included within-participant comparison. After obtaining informed parental consent, infants were randomized (by drawing a sealed, numbered envelope) to start the study on either neonatal helmet CPAP or nasal CPAP. Each infant was studied for two subsequent 90-min periods, alternating between neonatal helmet CPAP and nasal CPAP. Before every recording period a 15-min period was left for changing the device and stabilizating the patient. For each device a 10-min period for changing the device and an initial 5-min treatment were allowed before recording data. The level of CPAP was kept constant regardless of which device was being used. The FIO2 was adjusted to maintain the transcutaneous oxygen saturation (tcSaO2) in the range 92–96%. If the infant’s condition deteriorated significantly (persistent heart rate <80 bpm, and tcSaO2 <85% lasting for more than 3 min, not responding to increasing FIO2 or stimulation), it was allowed to the attending clinician whether to discontinue CPAP and to institute mechanical ventilation. The infants were nursed in their isolettes in a thermoneutral environment, and care was continued as previously. All infants were fed continuously via an orogastric tube.
During the 3-h study CPAP level, FIO2, respiratory rate, heart rate, tcSaO2 (Agilent Technologies, Boblingen, Germany), tcPO2, and tcCO2 (Linde Medical Sensors, Basel, Switzerland) were monitored continuously (at 1-min intervals) and recorded on a computer. Blood pressure and the infant’s comfort were detected every 15 min. The infant’s comfort was scored using the NIPS by the attending nurse [13]. This scale was specifically designed for newborns and contains six categories of assessment: facial expression, crying, breathing patterns, arm movement, leg movement, and state of arousal. It has a high interrater reliability [13]. To determine interobserver variability 20 routine procedures were scored before the data collection period by the three nurses involved in the study; interobserver variability was 18%. Where possible the same nurse scored the baby during the entire study. The skin temperature was monitored at the start and end of each recording time. At the end of the study the recordings were analyzed, and the number of desaturations was recorded while the infant was receiving either neonatal helmet CPAP or nasal CPAP. A significant desaturation was defined as a tcSaO2 less than 85%.
Three hours before the recording period and 2 days within the end of the study we performed cranial ultrasonography (ATL Ultrasound System, Bothell, Wash., USA) to evaluate infants for the presence of subependymal or intraventricular hemorrhage [14]. The primary objective was to compare the neonatal helmet CPAP to the nasal CPAP in terms of the infant’s comfort. Secondary objectives were to evaluate the changes in oxygen requirements, respiratory rate, heart rate, tcPO2, tcPCO2, blood pressure, skin temperature, and desaturations.
Statistical analysis
The sample size was calculated on the assumption that a change in NIPS score of more than 50% would be clinically significant, and that the standard deviation of the change was likely to be of a similar size. Assuming a power of 80% and level of statistical significance at p=0.05, 24 patients would be required to detect the difference in a cross-over study. Data are expressed as mean ±SEM or median (range), as appropriate. Results were compared by paired t test for within-subject comparisons. Wilcoxon matched pairs test was used when paired data were not normally distributed. To evaluate the time course of the signals the data were estimated at baseline, and subsequently they were averaged every 30 min. A p value less than 0.05 was considered statistically significant. The study was approved by the Padua University Ethics Committee.
Results
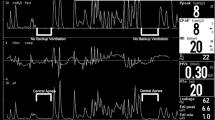
Calibration against an independent oxygen analyzer showed that the nasal CPAP system delivered 0.4% more oxygen than neonatal helmet CPAP. The average NIPS scores were significantly lower during the overall measuring period when the infants were on the neonatal helmet CPAP than when they were on nasal CPAP (0.26±0.07 vs. 0.63±0.12, p<0.01). No differences were found between the two CPAP treatments in terms of average values of CPAP level, FIO2, respiratory rate, heart rate, tcSaO2 tcPO2, tcCO2, blood pressure, or temperature (Table 2). The number of desaturations (18 vs. 32, p=0.09) was decreased, but not significantly during the period when the patients were on neonatal helmet CPAP. During the study period NIPS scores were significantly lower when the patients where in the helmet CPAP than in nasal CPAP treatment at 30, 60, and 90 min (Fig. 2). No differences were found on the other parameters (CPAP level, FIO2, respiratory rate, heart rate, tcSaO2 tcPO2, tcCO2, and blood pressure) between the two CPAP treatments at baseline, 30, 60, or 90 min. No local damage was noted in any of the infants during the study.
Discussion
The purpose of this study was to evaluate the effectiveness of the neonatal helmet CPAP as an alternative to a conventional nasal CPAP for treating premature infants needing continuous distending pressure. Data from our a 3-h cross-over study show that the neonatal helmet CPAP improves the clinical comfort in treated patients compared to that with conventional nasal CPAP.
Gregory et al. [4] were the first to describe two methods of delivering CPAP for treating RDS: through an endotracheal tube and through a plastic pressure chamber around the infant’s head. In this study, for the first time, we used CPAP delivered by a new device (neonatal helmet CPAP) to treat preterm infants. In comparison with the study by Gregory et al. [4], in which “the infant’s head was enclosed in the chamber with a loosely fitting collar about the neck,” our “new CPAP device” leaves the neck free, and the pressure in the system is guaranteed by a membrane that lies on the patient’s shoulders. This avoids the “garrotting” effect of the collar and consequently the risk of cerebral hemodynamic complications, such as intraventricular hemorrhage and hydrocephalus [15]. It is conceivable that differential atmospheric pressure on the neck vessels vs. thorax could produce the same effect; however cranial ultrasound evaluations in our patients were unchanged after the neonatal helmet CPAP treatment. We appreciate that, even with this information, the numbers were far too small to evaluate this possible complication; however, in a recent study we showed that cerebral perfusion does not differ between patients treated with this new device and those treated with the conventional nasal CPAP [16].
Factors determining the effectiveness of any nasal CPAP device include its associated work of breathing, flow characteristics, ease of use, and comfort of the infant once the device is in place [5, 6]. Although differences in performance have been reported between the different nasal CPAP devices, results from clinical studies are not conclusive [5, 6, 17, 18, 19, 20, 21, 22].
The most important objective of our new CPAP system design is the improvement in the patient-ventilator interface for achieving the greatest tolerability of patients receiving CPAP therapy. These advantages have been demonstrated in adult patients with acute hypoxemic respiratory failure using a similar device (helmet) for administering noninvasive pressure support ventilation [8]. Studies assessing the level of comfort in newborns have focused on three major areas: behavioral reactions, changes in physiological variables, and variation in hormonal or biochemical responses. Based on the NIPS, a scale that has been established for reliability and validity [13], our results suggest that neonatal helmet CPAP is better tolerated than conventional nasal CPAP during the entire recording period. Among the three nurses involved in the study the interobserver variability, a parameter that could influence the interpretation of the data, was low. On the other hand, unfortunately, the study was not blind and one could argue that the attending nurse was influenced in scoring the infant’s comfort. While this hypothesis is reasonable, the well-being of the patients during the neonatal helmet CPAP treatment was also confirmed by other, more objective physiological parameters, such as heart rate, blood pressure, oxygenation, and episodes of desaturation, although the differences were not statistically significant.
The decrease in the number of desaturations recorded while infants were receiving neonatal helmet CPAP, although not statistically significant, could have a clinical relevance and be a subject for a longer, larger study. FIO2 adjustments were made during the entire recording periods, suggesting that the desaturations were not related to the change in the device. The more stable method of fixation of the neonatal helmet CPAP device and the complete elimination of the air leakage around the prongs and secondary to mouth opening could explain this difference. It is noteworthy that the most difficult aspects of using nasal CPAP are positioning and managing the device [6]. On the other hand, it should be borne in mind that in cases of excessive pressure the mouth would act as a natural pop-off valve, possibly reducing the incidence of pneumothorax [5].
Limitations of this investigation include the fact that it was not a randomized controlled study; due to safety reasons clinically stable patients were enrolled for only a short-term physiological study. However, the design of this trial, with randomization of the first technique of CPAP and the cross-over method using within-subject comparison meant that any carry-over effect from one technique was balanced out. From a practical point of view, the CPAP would be interrupted at any time that the patient requires nursing care since the neonate is enclosed in a chamber. This was likely not to be required during the brief study period and with the relatively healthy babies in this protocol, but it could be a problem with longer use and more severely ill infants. Although we observed no short-term complication or potential problem over time with this new method, the size and the design of this study do not permit conclusions to be drawn regarding the long-term effectiveness or limitations of this new device. After this pilot study was conducted in stable patients, it would be very interesting to determine whether the same results could be obtained in sicker infants or during a longer period of time.
In conclusion, the neonatal helmet CPAP appears to be a feasible method of supporting the breathing of preterm infants. It seems to guarantee a better tolerability and at least similar improvement in oxygenation compared with conventional nasal CPAP. However, this is a short-term physiological study, and the results may not be valid for infants that are maintained on CPAP for days. A much larger randomized, controlled trial is needed to demonstrate the real advantages of this new device with respect to the other conventional techniques.
References
Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB (1987) Is chronic lung disease in low birth weight infants preventable? A survey of eight centres. Pediatrics 79:26–30
Van Marter L, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, Susser M, Paneth N, Leviton A (2000) Do clinical markers of barotraumas and oxygen toxicity explain interhospital variation in rates of chronic lung disease? Pediatrics 105:1194–1201
Davis PG, Henderson-Smart DJ (2002) Nasal continuous positive airway pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Library, issue 1, Oxford
Gregory GA, Kitterman JA, Phibbs RH, Tooley WA, Hamilton WK (1971) Treatment of the idiopathic respiratory distress system with continuous positive airway pressure. N Engl J Med 284:1333–1340
Polin RA, Sahni R (2002) Newer experience with CPAP. Semin Neonatol 7:379–389
De Paoli AG, Morley C, Davis PG (2003) Nasal CPAP for neonates: what do we know in 2003? Arch Dis Child Fetal Neonatal Ed 88:F168–F172
Robertson NJ, McCarthy LS, Hamilton PA, Moss ALH (1996) Nasal deformities resulting from flow driver continuous positive airway pressure. Arch Dis Child 75:F209–F212
Antonelli M, Conti G, Pelosi P, Gregoretti C, Pennisi MA, Costa R, Severgnini P, Chiaranda M, Proietti R (2002) New treatment of acute hypoxemic respiratory failure: noninvasive pressure support ventilation delivered by helmet-A pilot controlled trial. Crit Care Med 30:602–608
Chiumello D, Pelosi P, Carlesso E, Severgnini P, Aspesi M, Gamberoni C, Antonelli M, Conti G, Chiaranda M, Gattinoni L (2003) Noninvasive positive pressure ventilation delivered by helmet vs. standard face mask. Intensive Care Med 29:1671–1679
Patroniti N, Foti G, Manfio A, Coppo A, Bellani G, Pesenti A (2003) Head helmet versus face mask for non-invasive continuous positive airway pressure: a physiological study. Intensive Care Med 29:1680–1687
Cavaliere F, Conti G, Costa R, Proietti R, Sciuto A, Masieri S (2004) Noise exposure during noninvasive ventilation with a helmet, a nasal mask, and a facial mask. Intensive Care Med 30:1755–1760
Moa G, Nilsson K (1993) Nasal continuous positive airway pressure: experiences with a new technical approach. Acta Paediatr 82:210–211
Lawrence J, Alcock D, McGrath P, Kay J, MacMurray S, Dulberg C (1993) The development of a tool to assess neonatal pain. Neonatal Netw 12:59–66
Papile LA (1997) Intracranial hemorrhage. In: Fanaroff AA, Martin RJ (eds) Neonatal-perinatal medicine, chap 37. Mosby-Year Book, St. Louis, pp 891–899
Vert P, Andre M, Sibiout M (1973) Continuous positive airway pressure and hydrocephalus. Lancet II:319
Zaramella P, Trevisanuto D, Freato F, Quaresima V, Saraceni E, Chiandetti L (2004) Does Helmet-CPAP reduce cerebral blood flow by comparison with nasal CPAP in very low birth weight newborns? Pediatr Res 55:414A
Courtney SE, Pyon KH, Saslow JG, Arnold GK, Pandit PB, Habib RH (2001) Lung recruitment and breathing pattern during variable versus continuous flow nasal continuous positive airway pressure in premature infants: an evaluation of three devices. Pediatrics 107:304–308
Klausner JF, Lee AY, Hutchinson AA (1996) Decreased imposed work with a new nasal continuous positive airway pressure device. Pediatr Pulmonol 22:188–194
De Paoli AG, Davis PG, Faber B (2002) Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Library, issue 4, Oxford
Stefanescu BM, Murphy WP, Hansell BJ, Fuloria M, Morgan TM, Aschner JL (2003) A randomized, controlled trial comparing two different continuous positive airway pressure systems for the successful extubation of extremely low birth weight infants. Pediatrics 112:1031–1038
Goldman SL, Brady JP, Dumpit FM (1979) Increased work of breathing associated with nasal prongs. Pediatrics 64:160–164
Hammer J (2001) Nasal CPAP in preterm infants—does it work and how? Intensive Care Med 27:1689–1691
Acknowledgements
We thank Miss Pasqualina Simioni and the neonatal intensive care nurses of the Pediatric Department, University of Padua for their help during the study. Their continuous support and devotion to the care of our patients is greatly appreciated. We thank Dr. Regana Contini for revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trevisanuto, D., Grazzina, N., Doglioni, N. et al. A new device for administration of continuous positive airway pressure in preterm infants: comparison with a standard nasal CPAP continuous positive airway pressure system. Intensive Care Med 31, 859–864 (2005). https://doi.org/10.1007/s00134-005-2638-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2638-9