Abstract
Objective
A goal-directed therapy algorithm based on serial lactate values obtained from a point-of-care testing device was utilized in an attempt to reduce the mortality of patients after congenital heart surgery.
Design
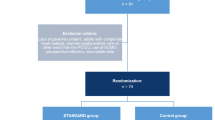
Prospective study of patients undergoing surgery utilizing a goal-directed therapy algorithm in the postoperative period. The results of this group are compared with a historical cohort. Operative risk was determined using the RACHS-1 scoring system.
Setting
A 12-bed cardiac intensive care unit (ICU) in a pediatric hospital.
Patients
Patients undergoing surgery from July 2001 through September 2003 (group B, n=710) were compared to cohorts from June 1995 through June 2001 (group A, n=1,656). Group B patients were smaller and younger (median weight 6.2 vs 8 kg, p<0.001; median age 184 vs 327 days, p=0.004).
Interventions
Beginning in July 2001, blood lactate measurements were performed serially for 24 h after heart surgery. Based on lactate values and trends, therapy was amended.
Measurements and results
Mortality was lower for group B (1.8 vs 3.7%, p=0.02). A reduction in mortality between group B and group A was noted in neonates (3.4 vs 12%, p=0.02), but not in older patients. Group B patients undergoing higher risk operations (Risk Adjustment for Congenital Heart Surgery-1 [RACHS-1] categories 3–6) had a significant reduction in mortality when compared to group A (3 vs 9%, p=0.006), no difference was noted in patients undergoing lower risk operations (RACHS-1 categories 1 and 2).
Conclusions
The combination of goal-directed therapy and point-of-care testing was associated with a marked decrease in mortality for patients undergoing congenital heart surgery. Improvement was greatest in the highest risk patients.
Similar content being viewed by others
Introduction
While mortality for children undergoing congenital heart surgery (CHS) continues to diminish, it remains substantial for certain high risk operations and neonatal palliations. Surgical modifications have had significant impact on mortality, but intensivists must continue to find ways to improve outcomes in the highest risk patients.
Goal-directed therapy (GDT) has also been shown to substantially improve patient outcome in critical illness [1–5]. GDT utilizes an algorithm to modify therapy in order to achieve a predetermined goal. Variables are measured at regular intervals to monitor progress. When the variables are not improving satisfactorily, therapy is escalated to achieve the desired goal.
Point-of-care testing (POCT) or near-patient testing is defined as performing any laboratory test at or near the site of patient care. POCT is usually associated with a rapid turn around time (TAT), allowing clinicians to make important decisions in prompt fashion. This should result in improved outcomes [6, 7].
The influence of both POCT and GDT on the outcomes of pediatric cardiac surgical patients has never been evaluated. We believed that blood lactate could become a treatment end point for our relatively homogeneous group of patients (those suffering from or potentially suffering from low cardiac output syndrome after heart surgery). The short TAT for obtaining lab results via a POCT device made it possible for us to develop a GDT algorithm based on blood lactate values. We assumed the combination of POCT and GDT aimed at normalizing blood lactate levels would increase survival after heart surgery. In this study we evaluated the impact of the combination of these changes on the mortality rate in pediatric cardiac surgical patients.
Patients and methods
Patients
All patients undergoing cardiac surgery in a 268-bed free-standing Children’s Hospital from June 1995 through September 2003 were included in the study. Patients were recovered in a 12-bed cardiac intensive care unit (CICU). Care was rendered by a multidisciplinary cardiovascular team.
The outcomes of all patients undergoing CHS from July 2001 through September 2003 (group B) were compared to historical cohorts operated on from June 1995 through June 2001 (group A). There were 1,656 patients in group A and 710 patients in group B. Patients in group B were smaller and younger than those in group A ( median weight 6.2, range 0–114 kg vs 8, range 0–127 kg, p<0.001; median age 184, range 0–19,746 days vs 327, range 0–26,423 days, p=0.004). Neonates accounted for 29% of group B patients vs 19% of group A patients. A total of 64% of group B patients were less than 1 year of age at the time of surgery, vs 51% of group A. Patient demographics are shown in Table 1.
Study design
Near-patient testing in the form of a POCT device, the i-Stat analyzer (Abbott) was introduced to the CICU in July of 2001. The analyzer is a hand-held device, which uses cartridges to perform a variety of laboratory tests. Twenty microliters of blood are instilled into the cartridge, which is then inserted into the analyzer. Subsequently the result is displayed on the analyzer’s screen, printed out and reviewed by the team members. The authors received no support, financial or otherwise, for the development or implementation of this project.
From July 2001 through July 2003, serial arterial blood lactate levels were measured in all postoperative patients. An algorithm was developed to direct physician response to changing lactate levels (Fig 1). Routine measurement of blood lactate was discontinued when the blood lactate level returned to normal (<2.2 mmol/l for the purpose of our study). In neonates, blood lactate was measured hourly for the first 4–6 h after admission to the CICU. If the lactate level was less than 5 mmol/l or if the lactate trend was acceptable (decrease of more than 0.5 mmol/l per h), lactate was measured every 4–6 h until normal. For all other patients lactate was measured serially every 4–6 h. The frequency of serial lactate monitoring could be increased at the physician’s discretion if the clinical situation warranted it. Medical management of a patient was escalated if his/her blood lactate was noted to be rising or if the fall in lactate was less than 0.5 mmol/l per h. Escalation of medical management was individualized for each patient based on the attending physician’s or physician extender’s perception on how best to improve systemic oxygen delivery or diminish oxygen consumption (improve the ratio of oxygen delivery to oxygen consumption).
Outcome data for patients undergoing POCT was collected prospectively for later review. The outcome data was collected and stored utilizing the CardioAccess database (CardioAccess, Fort Lauderdale, Fl).
The operative risk for all patients undergoing heart surgery was determined according to the RACHS-1 (Risk Adjustment for Congenital Heart Surgery [8]) scoring system. RACHS-1 divides the surgeries into six categories, with category 1 being the lowest mortality operations and category 6 being the surgeries associated with the highest mortality.
Statistical analysis was performed using Sigma Stat for Windows Version 2.03, SPSS (Chicago, IL). Chi Square analysis was used to detected differences in mortality between groups. Mann Whitney rank sum analysis was used to determine differences in demographic data between groups.
Results
Risk Adjustment for Congenital Heart Surgery-1 scores were similar between the groups. The patients in group B had significantly longer cardiopulmonary bypass times and aortic cross-clamp times (Table 1).
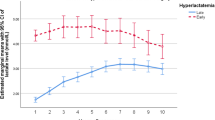
Figure 2 demonstrates mortality for patients undergoing surgery as related to age in groups A and B, and for the 27 months prior to July 2001 (27 months before group B). We believed the 27 months prior to beginning our GDT protocol would provide the most similar cohort to the 27 months of group B data reported. Mortality for all the patients in group B was lower than that for patients in group A (1.8 vs 3.7%, p<0.02). Mortality for the group of patients undergoing CHS in the 27 months prior to the introduction of i-Stat was 4.4%, nearly identical to group A patients. Comparing groups A and B, no difference in mortality was noted in patients older than 1 year, but a very significant difference was present in patients less than 1 year (p=0.008). Neonatal mortality was also significantly decreased for patients in group B (p=0.001). The yearly neonatal mortality for patients undergoing CHS is shown in (Fig. 3). There was no evidence of declining neonatal surgical mortality prior to the introduction of the i-Stat GDT protocol.
The patient outcomes were also grouped according to the risk of the surgery performed (RACHS-1 scores). This is shown in Fig.4. The patients in RACHS-1 categories 1 & 2, 3 & 4 and 5 & 6 were grouped together. Grouping was performed because the number of patients in each individual RACHS-1 category was sometimes small, making statistical comparison between groups A and B difficult. In the lower risk RACHS 1 & 2, there is no difference in mortality between groups A and B. However, in RACHS 3 & 4 and in 5 & 6, group B has significantly lower mortality than group A. When patients are grouped into low risk (RACHS-1 categories 1 and 2) and higher risk groups (RACHS-1 categories 3–6), a very significant decrease in mortality is noted for patients in group B undergoing higher risk surgery. Mortality for group A patients undergoing higher risk surgery was 9% as compared to 3% in group B patients (p=0.006).
Discussion
In July of 2001, the i-Stat blood gas analyzer was introduced to the CICU at Miami Children’s Hospital. The i-Stat was intended to replace most of the functions provided by our “stat lab” and allow us to measure blood lactate levels with a rapid TAT. The decision to incorporate the i-Stat into our practice was based on the following premise. First, we wanted to establish a practice that incorporated near-patient testing associated with the shortest possible TAT of essential laboratory data for managing patients after heart surgery. The TAT for critical lab data is 120 s for the i-Stat analyzer. Our belief was that this would allow the clinician to react promptly to changing physiologic conditions. Second, we elected to establish lactate as an objective indicator of oxygen debt. Investigators have suggested that the duration of time where the lactate remains elevated (so called “lactime”) is more important than a single lactate value in predicting mortality [9]. These investigators stressed the importance of serial lactate measurements. Our goal was to attempt to minimize “lactime” by establishing clinical guidelines directed at normalizing blood lactate levels. We believed that the combination of these changes in clinical practice would result in improved survival for patients undergoing CHS.
With the addition of the POCT device, a therapeutic strategy was devised using an algorithm based on serial lactate levels to guide medical therapy. Rising or elevated lactate was assumed to be secondary to diminished systemic oxygen delivery in this patient population. Based on the blood lactate level, medical therapy was escalated, unchanged or diminished (Fig. 1). Methods of escalating medical therapy differed from patient to patient and were determined by the patient’s attending physician based on other available clinical data. Treatment was individualized for each patient and might include transfusion to increase oxygen content of blood, escalation of inotropic support, initiation of additional inotropic agents, augmenting or adding systemic afterload reduction, changing ventilator settings or any maneuver thought best to increase systemic oxygen delivery. Clinicians might also choose to diminish oxygen consumption with sedatives, analgesics or the use of neuromuscular blockade. If the lactate trend was downward, the medical therapy was continued and then slowly decreased. If, in spite of maximal medical therapy, the blood lactate level was increasing or persistently high (more than 10), then this was used as an indication to initiate mechanical cardiopulmonary support.
The combination of a GDT-based postoperative management protocol and POCT resulted in a marked decrease in mortality for patients undergoing CHS in our institution. This decrease in mortality was achieved despite the fact that patients in group B were younger, smaller and undergoing longer and higher risk operations. It seems unlikely that the decrease in mortality was related to a generalized trend of diminished mortality for children undergoing CHS. The data for the 27 months prior to the introduction of our GDT protocol fails to indicate any perceptible improvement in outcomes (Fig. 2).
Lactate can reflect the adequacy of systemic oxygen delivery in critical illness [10, 11]. The adequacy of systemic oxygen delivery is dependent on both the magnitude of oxygen delivery and the metabolic demands of the patient. In patients recovering from CHS, elevation of lactate is most often related to an imbalance in the relationship of oxygen delivery to oxygen consumption. This imbalance can be complicated by liver and renal dysfunction (the organs primarily responsible for the metabolism of lactate). Improving tissue oxygen delivery in this population may, therefore, both diminish the production of lactate and improve the metabolism of lactate, resulting in a decrease in blood lactate.
Blood lactate has been used for years to reflect cardiac output, to assess the relationship of oxygen delivery to oxygen consumption and to help predict outcomes in high risk patients [12–14]. Lactate also reflects hemodynamic changes rapidly. Acute decreases in oxygen delivery are associated with a rapid increase in blood lactate level. Likewise, restoration of normal oxygen delivery results in a rather prompt restoration of blood lactate to normal levels [15].
Lactate monitoring has been shown to predict outcome in patients after CHS. Charpie evaluated neonates after complex CHS and found that poor outcome was associated with both an elevated initial lactate and an increase in lactate of 0.75 mmol/l per h [12]. High plasma lactate values in neonates with hypoxemia have been shown to be predictive of adverse neurodevelopmental outcome [16].
In goal-oriented or GDT, medical management is augmented or altered until the treatment goal (or resuscitation end point) is reached (in our study, until lactate had normalized or begun to trend downward) [17, 18]. Therapy directed at resuscitation end points, such as mixed venous oxygen saturation (SvO2), blood lactate concentration, pH and base deficit, remain the cornerstone of GDT in the critically ill.
The efficacy of GDT in the critically ill has been the subject of numerous clinical and experimental studies and meta-analyses [1–5, 19–21]. Shoemaker documented that there was a strong relationship between the duration and magnitude of oxygen debt and outcomes in high risk surgical patients [22]. He speculated that increasing oxygen delivery would minimize or prevent oxygen debt and improve outcomes. Rivers, in a prospective study of patients with severe sepsis/septic shock, attempted to diminish mortality with a GDT protocol [1]. The GDT group was noted to have significantly lower mean lactate concentration, higher pH, less severe organ dysfunction and markedly improved survival.
In a meta-analysis of high risk patients, the effect of hemodynamic optimization on outcome was analyzed [21]. After review of 21 studies, the authors concluded that early hemodynamic optimization resulted in a statistically significant decrease in mortality. The difference was more pronounced in those most severely ill. These findings are consistent with ours. By augmenting systemic oxygen delivery to achieve predetermined blood lactate goals, survival in our patients was increased most in those undergoing the highest risk operations (RACHS-1 categories 3–6) and in those thought to be the highest risk age group (neonates).
Despite mounting evidence suggesting GDT can improve outcome in critically ill adult patients, there is a scarcity of literature supporting its use in critically ill children. Rossi used mixed venous oxygen saturations (SvO2) to guide therapy in patients after the Norwood procedure [23]. Medical therapy was augmented or altered in an attempt to optimize both the SvO2 and the ratio of systemic to pulmonary blood flow, with a resultant increase in survival.
Survivors of CHS are noted to have evidence of inadequate oxygen delivery that usually resolves within 24 h of surgery [12, 23–25]. Estimating the adequacy of oxygen delivery in this patient population remains difficult. Intracardiac shunting and small patient size makes techniques such as SvO2 monitoring or thermodilution cardiac output monitoring difficult, if not impossible, for many patients. Estimating oxygen delivery often requires the placement of additional intracardiac catheters or catheters placed in the superior vena cava (SVC). Since the SVC oxygen saturation is not a true SvO2, conclusions related to the adequacy of systemic oxygen delivery based on an SVC oxygen saturation must be viewed cautiously. Blood lactate sampling becomes an excellent, non-invasive indicator of adequate tissue oxygen delivery. Minimizing the period of oxygen debt has resulted in improved survival for patients who were recovering from major trauma [26]. We believed that therapy aimed at normalizing blood lactate levels as quickly as possible would lead to a shorter duration of oxygen debt, more rapid restoration of renal or liver dysfunction, fewer postoperative complications and improved survival.
As POCT technology improves, smaller and more portable instruments are devised to perform multiple tests in a short amount of time while requiring minimal or no calibration [6, 7, 27]. While POCT has shown to affect the outcome in disease states such as diabetes indirectly [28], improvement in outcomes has only occasionally been shown in critical care areas. Recently, newer near-patient devices which evaluate coagulation profiles after heart surgery have been shown to reduce transfusion requirements, but not mortality. [29, 30]. This, despite the intuitive concept that rapid TAT associated with POCT devices should allow clinicians to make critical decisions promptly in patients with rapidly developing clinical problems.
With the addition of the i-Stat POCT device to our CICU, lactate became our ideal end point of resuscitation. It required little additional expense and was measured with the same blood sample used for measuring routine arterial blood gas analysis. While the patient population in the CICU can be considered quite heterogeneous, evaluation of blood lactate almost always indicates a serious physiologic derangement, regardless of the patient’s underlying cardiovascular physiology. Unlike SvO2 monitoring, lactate levels have the same significance in single ventricle patients as they do in patients with biventricular physiology. A lactate level of 10 mmol/l always indicates serve physiologic derangement and increased risk for mortality. A SvO2 of 55% may represent ideal hemodynamics for a patient with single ventricle physiology or extreme low cardiac output for a patient with biventricular physiology.
The limitations of this study are that it was prospective, non-randomized and used an historical control group. Because of the study design, there is a possibility that the difference in outcomes might be due to factors other than the implementation of the GTD protocol. Since the control group had surgery in an earlier era, it is possible that other improvements in technology and equipment might have contributed to the lower mortality in the protocol patient group. Standard protocols for management of patients after CHS were followed in both eras and were not significantly altered with the exception of our GDT algorithm. The medications used for preoperative, intraoperative and postoperative management were similar in both eras. Operative techniques were not changed substantially during the course of the study period. In group B, four category 6 patients underwent a modification of the Norwood procedure with placement of a right ventricular to pulmonary artery conduit, two survived. The percentage of patients placed on ECMO did not differ between the groups. Changes in personnel did occur over the course of the study, including a change in intensive care leadership. The effects of these changes cannot be determined. Any clinical study occurring over an 8-year period would be associated with major changes in personnel. We believe the turnover in personnel had little impact on the outcomes.
In conclusion, critically ill patients represent a unique challenge to both laboratory and clinical services. Rapidly changing physiologic conditions warrant careful and prompt evaluation and treatment. For patients recovering after congenital heart operations, the combination of POCT and a GDT protocol based on serial blood lactate values was associated with a marked reduction in mortality. The reduction in mortality was greatest in both the youngest patients and those patients undergoing the highest risk procedures. It is possible that GDT protocols aimed at normalizing other objective indicators of cardiovascular well being may result in similar improvements in outcomes.
Refrences
Rivers E, Nguyen B, Haystad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M (2001) Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345:1368–1377
Boyd O, Grounds RM, Bennett ED (1993) A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 270:2699–2707
Polonen P, Ruokonen E, Hippelainen M, Poyhonen M, Takala J (2000) A prospective, randomized study of goal-directed hemodynamic therapy in cardiac surgical patients. Anesth Analg 90:1052–1059
Boyd, O, Hayes, M (1999) The oxygen trail: the goal. Br Med Bull 55(1):125–139
Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee T-S (1988) Prospective trial of supernormal values of survivors as therapeutic goals in high-risk surgical patients. Chest 94:1176–1186
Castro HJ, Oropello JM, Halpern N (1995) Point-of-care testing in the intensive care unit—the intensive care physician’s perspective. Am J Clin Pathol 104:S95–S99
Price CP (2001) Point-of-care testing—impact on medical outcome. Clin Lab Med 21:285–303
Jenkins JJ, Gauvreau K (2002) Center-specific differences in mortality: preliminary analyses using the risk adjustment in congenital heart surgery (RACHS-1) method. J Thorac Cardiovasc Surg 124:97–104
Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL (1996) Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg;171:221–226
Backer DD (2003) Lactic acidosis. Intensive Care Med 29:699–702
Luft FC (2001)Lactic acidosis update for critical care clinicians. J Am Soc Nephrol 12:S15–S19
Charpie JR, Dekeon MK, Goldberg CS, Mosca RS, Bove EL, Kulik TJ (2000) Serial blood lactate measurements predict early outcome after neonatal repair or palliation for complex congenital heart disease. J Thorac Cardiovasc Surg 120:73–80
Fuloria M (2002) Elevated plasma lactate levels: a tool for predicting outcomes or for improving care. Crit Care Med 30:2166–2167
Husain FA, Martin MJ, Mullenix PS, Steele SR, Elliott DC (2003) Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg 185:485–491
Leavy JA, Weil MH, Rackow EC (1988) ‘Lactate washout’ following circulatory arrest. JAMA 260:662–664
Da Silva S, Hennebert N, Denis R, Wayenberg JL (2000) Clinical value of a single postnatal lactate measurement after intrapartum asphyxia. Acta Paediatr 89:320–323
Porter JM, Ivatury RR (1998) In search of the optimal end points of resuscitation in trauma patients: a review. J Trauma 44:908–914
Elliott DC (1998) An evaluation of the end points of resuscitation. J Am Coll Surg 187:536–547
Bennett ED (2002) Goal-directed therapy is successful—in the right patients. Crit Care Med 30:1909–1910
Tighe D, Moss R, Heywood G, al-Saady N, Webb A, Bennett D (1995) Goal-directed therapy with dopexamine, dobutamine and volume expansion: effects of systemic oxygen transport on hepatic ultrastructure in porcine sepsis. Crit Care Med 23:1997–2007
Kern JW, Shoemaker WC (2002) Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med 30:1686–1692
Shoemaker WC, Patil R, Appel PL, Kram HB (1992) Hemodynamic and oxygen transport patterns for outcome prediction, therapeutic goals and clinical algorithms to improve outcome. Feasibility of artificial intelligence to customize algorithms. Chest 102:617S–625S
Rossi AF, Sommer RJ, Lotvin A, Gross RP, Steinberg LG, Kipel G, Gloinko RJ, Griepp RB (1994) Usefulness of intermittent monitoring of mixed venous oxygen saturation after stage I palliation for hypoplastic left heart syndrome. Am J Cardiol 73:1118–1123
Rossi AF, Seiden HS, Gross RP, Griepp RB (1999) Oxygen transport in critically ill infants after congenital heart operations. Ann Thorac Surg 67:739–744
Tweddell JS, Hoffman GM, Fedderly RT, Ghanayem NS, Kampine JM, Berger S, Jaquiss RD, Ghanayem NS, Frisbee SJ, Litwin SB (2000) Patients at risk for low systemic oxygen delivery after the Norwood procedure. Ann Thorac Surg 69:1893–1899
Blow O, Magliore L, Claridge JA, Butler K, Young JS (1999) The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. J Trauma.47:964–969
Widness JA, Kulhavy JC, Johnson KJ, Cress GA, Kromer IJ, Acarregui MJ, Feld RD (2000) Clinical performance of an In-Line Point-of-Care monitor in neonates. Pediatrics 106:497–504
Price CP (2003) Point-of-care testing in diabetes mellitus. Clin Chem Lab Med;41:1213–9
Samama CM, Ozier Y (2003) Near-patient testing of haemostasis in the operating theatre: an approach to appropriate use of blood in surgery. Vox Sang 84:251–255
Avidan MS, Alcock EL, Da Fonseca J, Ponte J, Desai JB, Despotis GJ, Hunt BJ (2004) Comparison of structured use of routine laboratory tests or near-patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth 92:178–186
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossi, A.F., Khan, D.M., Hannan, R. et al. Goal-directed medical therapy and point-of-care testing improve outcomes after congenital heart surgery. Intensive Care Med 31, 98–104 (2005). https://doi.org/10.1007/s00134-004-2504-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2504-1