Abstract
Objective
To identify routes and patterns of colonization with Pseudomonas aeruginosa in intubated patients to design strategies of prevention for respiratory infection.
Design and setting
Prospective and observational study in the 16-bed intensive care unit of a teaching hospital.
Patients and participants
Ninety-eight intubated patients were investigated over a 3-year period. Those ventilated less than 72 h were excluded.
Measurements and results
Samples from the tap water from each patient’s room, stomach, oropharynx, subglottic secretions, trachea, and rectum were collected when the patient was intubated, and then three times per week. Pulsed-field gel electrophoresis was performed to type the strains. We identified 1,607 isolates pertaining to 35 different pulsotypes. Overall 54.2% of patients presented colonization, and tracheal colonization was present in 30.5%. Ten patients had colonization at intubation, and four of these developed ventilator-associated pneumonia (VAP) after a mean of 4±2 days. ICU-acquired colonization occurred in 31 patients, and 4 of these developed VAP after a median of 10±5 days. P. aeruginosa was isolated from the room’s tap water in 62.4% of samples. More than 90% of tap water samples had pulsotypes 1 and 2, which were frequently isolated in the stomach (59%) but were only rarely associated with VAP.
Conclusions
Although colonization/infection with P. aeruginosa in intubated patients tends to be endogenous, exogenous sources should not be ruled out. A combination of early identification (and eradication) of airways colonization by P. aeruginosa plus infection control measures targeted to reduce cross-contamination should be the basis to prevent pulmonary infection.
Similar content being viewed by others
Introduction
Pseudomonas aeruginosa (PA) is highly endemic in intensive care units (ICUs), where it causes urinary tract infections, surgical wound infections, bacteremia, and pneumonia [1, 2]. According to the report of the National Nosocomial Infections Surveillance System [3], PA is the second most frequent pathogen in ICUs. Although colonization by PA frequently precedes overt infection, the origin of the organism and the precise mode of transmission are often unclear [4, 5]. The origin may be exogenous (the environment, other patients, or respiratory equipment) or endogenous (the oropharynx, stomach, or gut). Establishing the source of strains causing colonization is crucial for the development of effective preventive measures.
The main goal of this study was to identify the routes of colonization by PA in intubated patients and the sources of the strains causing colonization or respiratory infection over a long period and without an epidemic outbreak. The findings may have important implications for the design of evidence-based preventive measures for ventilator-associated pneumonia (VAP). A secondary goal was to determine whether patients were colonized or infected with a single or with multiple genotypes of PA, an issue that may have implications for diagnosis.
Material and methods
This prospective study was conducted over a 35-month period (1 April 1996 to 28 February 1999) in a medical-surgical ICU of a teaching hospital. The Human Subjects Review Committee of the Hospital approved the study protocol (96-027).
Study population
The study originally enrolled 98 patients, 26 of whom were later excluded because the duration of MV was less than 72 h; thus 72 patients were finally evaluated (Table 1). All patients intubated with Hi-Lo Evac (Mallinckrodt Laboratories, Athlone, Ireland) were eligible; patients with tracheotomies and different tubes were not included. A maximum of three patients were evaluated simultaneously. The nurse-patient ratio was 1:2. The Acute Physiology and Chronic Health Evaluation II score [6] was used to assess the severity of illness at admission. The infection control measures used in the ICU during the study period consisted of hand hygiene with clorhexidine, use of gloves, and cleaning once daily with tap water of the sink and environment surfaces using glutaraldehid solutions. Eight patients died during the study.
Surveillance cultures
Samples from gastric aspirate, oropharyngeal swab, subglottic secretions, internal surface of endotracheal tube, tracheal secretions, and rectal swab were taken in each patient after intubation and then three times per week. Tap water from the individual ICU room was also cultured at admission and every 72 h. Follow-up was considered complete at 21 days of mechanical ventilation (MV) or when the patient was extubated, a tracheotomy was carried out, the patient died, or an episode of VAP by PA was diagnosed. Surveillance cultures from environmental surfaces of the ICU and from the hands of health care workers (HCW) were taken on five separate days during the study period, without prior warning.
Definitions
Colonization was defined as the isolation of PA from specimens taken from any body site studied in the absence of infection. Colonization at intubation was defined as that occurring within 24 h of entry into the study. When colonization occurred more than 48 h after entry in the study, it was defined as ICU acquired. The trachea was considered as the initial site of colonization when no prior colonization was documented at other sites. The routes of PA colonization leading to the trachea were determined by analyzing the chronological comparison of the pulsotypes (PTs) obtained from the different samples.
Primary or endogenous colonization was defined as colonization by a strain of PA that had not been previously isolated from another studied patient, the hands of HCW, or another site analyzed in the ICU environment. Exogenous colonization was defined as colonization by a strain of PA with a PT previously isolated from another studied patient, hands of HCW, or another environmental surface in the ICU. Diagnosis of VAP has been reported elsewhere and confirmed by a positive protected specimen brush culture containing at least 103 colony-forming units (CFU)/ml, a positive bronchoalveolar lavage culture with at least 104 CFU/ml or by quantitative endotracheal aspirate with 106 CFU/ml or more [7, 8].
Microbiological and bacterial typing
Hands of HCW were studied by the rinse sampling method [9]. All surfaces studied and taps were studied by the wipe-rinse sampling method or the swab-rinse sampling method [9]. Samples were plated in cetrimide agar plates and in nonselective broth. Pharyngeal swab, gastric aspirate, subglottic secretions, tracheal aspirate, endotracheal tube, and rectal swab of each patient were sampled on cetrimide agar plates and chocolate agar plates, and the cultures were reported semiquantitatively. Whenever possible at least four colonies representative of the different morphological types of PA present on the culture plate were picked for subsequent characterization beyond species level.
Pulsed-field gel electrophoresis (PFGE) was performed using a previously described method [10]. Electrophoresis was performed with a Chef DRIII System apparatus (Bio-Rad, Richmond, Calif., USA) under conditions appropriate for the enzymes. Analysis of PFGE profiles was made with the software Bio Image Whole Band Analyzer (Genomic Solutions, Ann Arbor, Mich., USA).
Each strain was first compared with all others to calculate similarity using the Dice correlation coefficient (SD). SD was defined as twice the number of shared bands divided by the total number of bands. Isolates were defined as belonging to the same clonal lineage if their patterns exhibited less than six band differences (two genetic events) [11]. The most common profile among the isolates of each lineage was designated as the parental profile. Numbers designated strains corresponding to each major macrorestriction type, and a letter suffix indicated each of their subclonal variants (subtypes). The patterns were ordered chronologically according to time of collection. All isolates were first digested with XbaI. Moreover, a representative strain of the different PTs and subtypes were subjected to further digestion with a second enzyme (DraI). The final types and subtypes are the result of the combined two-enzyme analysis.
Statistical analysis
Descriptive analysis was performed. Continuous variables were expressed as means (±SD). Associations of categorical variables were assessed with the χ2 test or with Fisher’s test.
Results
In 39 (54.2%) PA colonization could be proven during the study period (Fig. 1). Ten patients (13.9%) were already colonized at intubation. Two of these acquired different PA strains during the study period. Thus 31 patients (43%) were colonized during the period of MV and were classified as ICU acquired.
Genotyping
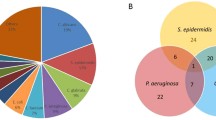
When available, four colonies were subcultured and independently typed, and a total of 1,612 isolates were obtained; 5 of these could not be studied by PFGE. Thirty-five different PFGE patterns or pulsotypes (PTs) were found. Among 279 cultures performed from the five environmental surveillance cultures 41 (14.7%) were positive for PA, and 15 different PTs were identified: the most frequent were PT1 (31.7%), PT7 (17.1%), and PT8 (14.6%). The cultures from the inanimate surfaces of the ICU were positive for PA in 34.3% of samples and the hands of HCW in 6% of cases. On the other hand, 93 of 149 (62.4%) cultures from the tap water of patients’ rooms were positive for PA. Two PTs were identified in 26 cultures. Eleven different PTs were identified among PA cultures isolated from tap water, PT2 (74.2%) and PT1 (32.3%) being the most frequently isolated.
In the 39 colonized patients 288 positive cultures for PA were obtained. In this case, 22 different PTs were obtained, PT2 (51.3%) and PT1 (41%) being the most frequently isolated. The distribution of PTs differed significantly in the stomach than in other sites (Fig. 2).
Of the cultures analyzed 13% were polyclonals, 24.8% of environmental and tap water cultures, but only 7.3% of those from the various patient sites (p<0.05). The stomach (19.1%) and rectum (14.3%) had the highest incidence of polyclonals. Only one of the lower respiratory tract cultures was polyclonal.
Pseudomonas aeruginosa colonization
Ten of the 39 patients with PA (25.6%) were already colonized at intubation. Eight had prior hospitalization, and three were intubated after the day of admission in the ICU. Two had multiple PTs. Seven of the 13 strains of PA isolated in this subset (53.8%) had primary colonization (single PFGE pattern). The remaining six strains (46.2%) were considered exogenous colonization (three from tap water). Detailed information on distribution is shown in Table 2. Only four strains were initially isolated from the digestive tract (stomach or rectum).
Most of the 31 patients who suffered ICU-acquired PA colonization during ventilation presented simultaneous colonization at multiple sites. Distribution of colonization and days of colonization is shown in Table 3. Among the 31 patients with ICU-acquired colonization 47 strains of PA were isolated. Eighteen patients were colonized with a single PT. Of the 47 strains of PA 39 (83%) were classified as exogenous. The stomach was the site with the highest incidence of exogenous colonization (94% of strains). PT1 and PT2 isolated from the tap water and environment were the most frequent PTs colonizing patients with ICU-acquired colonization. Excluding these PTs, the majority of the remaining PTs were considered endogenous. Detailed information on body sites colonized and PTs of PA in this group of patients is shown in Fig. 3 and Table 3.
Most of patients included in the study (94.4%) received antibiotic treatment prior to PA colonization. Sixteen patients (22.2%) were treated with third-generation cephalosporins, and 35 of 72 patients (48.6%) received antibiotics affecting PA. Only the prior treatment with third-generation cephalosporins was associated with a risk of colonization with PA (p=0.03).
Respiratory colonization
In our study 22 of 72 patients (30.5%) had colonization of the lower respiratory tract, 5 (6.9%) at intubation. Among the five patients colonized at the onset of MV four were primary. In contrast, 14 of 17 PTs isolated from the 17 patients with ICU-acquired tracheal colonization were of exogenous origin. Eight of these 17 PTs simultaneously colonized airways and gut; in three cases the first site colonized was the stomach. Only one patient had two different PTs.
Ventilator-associated pneumonia by Pseudomonas aeruginosa
Eight patients (11.1%) presented VAP caused by PA. Seven were confirmed by protected specimen brush and one by quantitative tracheal aspirate. The mean period of MV prior to the VAP onset was 7±4.7 days (range 3–17). Only two patients presented VAP within the first 4 days of MV. Among the five patients with upper respiratory tract colonized at onset of MV, four had VAP with 4±2 days (range 3–7) of intubation. The remaining four episodes of VAP were diagnosed in patients with ICU-acquired colonization, and the mean period of MV prior to the diagnosis of VAP was 10±5 days (range 6–17). The respiratory tract in seven of eight patients with VAP had been colonized previously by the same strain causing the pneumonia. The initial site of colonization for these strains was the upper airways in seven of eight patients (87.5%), but in four cases simultaneous digestive tract colonization was found. PFGE typing identified neither the stomach nor the rectum as the only initial site of colonization in any case. The mean period of previous tracheal colonization before the diagnosis of pneumonia was 4.3±3.4 days (range 2–11). In four cases (50%) the origin of strains causing VAP was considered exogenous (one tap water). In the remaining four patients with VAP the strains causing infection were considered primary.
Discussion
This study is unique in evaluating more than 1,600 isolates over a long period or time. More than one-half of intubated patients were colonized with PA, and this colonization was not limited to the respiratory tract. An important finding was that the source of strains causing ICU-acquired colonization was predominantly environmental, in most cases the tap water from the patient’s room. However, this colonization was mainly in the gastrointestinal tract and did not affect the clinical course of the patients, because our results showed that the strains causing respiratory infections were endogenous in one-half of VAP episodes, emphasizing the limitations of infection control measures to prevent VAP by PA.
The incidence rate of colonization by PA in a range the ICU ranges between 4.5% and 37% [5, 12, 13, 14]. Our incidence was higher, although comparison of incidence rates with those other ICUs is difficult, mainly because of differences in patient populations and in the samples analyzed. Indeed, in our study among only the patients with prolonged MV (>72 h) the incidence rate of colonization by PA was 54.2%. Differences in patients who were already colonized at intubation, colonization pressure, and number of samples may explain these differences [15].
The stomach showed the highest incidence (84%) of ICU-acquired colonization, and this location was the earliest site of colonization in most of the patients. Previous studies [5, 16] have suggested that the origin of digestive colonization was endogenous, but they did not simultaneously analyze patients’ samples and environmental and tap water cultures, nor did they subculture and independently type four colonies. In our study more than 60% of the tap water in the ICU was contaminated by PA, mainly PT1 and PT2, the PTs that predominated among strains isolated from the stomach. These results suggest that the origin of gastric colonization by PA is predominantly exogenous, and that PA was transmitted directly through the water infused in the stomach by gastric tube or indirectly via handling by the HCW after handwashing. Tap water and sinks have been recognized as the origin of outbreaks of respiratory and urinary infections in pediatric and adult ICUs due to PA [17, 18, 19].
Our findings agree with a study carried out in a surgical ICU [17]. A follow-up study in the same surgical ICU [20] confirmed that all taps in the patients’ rooms were contaminated with PA, and in 32% of cases a tap water isolate from the room was shown to be of the same genotype as the patient’s isolate. Our findings suggest that in our ICU the tap water was the source of acquisition because in most cases the detection of PA took place prior to the detection of a strain in the patient, and because the tap water was colonized mainly by the same PTs (1 and 2) whereas the pattern of cultures of other environmental surfaces of the ICU and patient samples was more heterogeneous.
Our results indicate that the clinical importance of gastrointestinal colonization by PA in mechanically ventilated patients is low. Indeed, although most of our ventilated patients presented a gastric ICU-acquired colonization, we found that tracheal ICU-acquired colonization was present in only 23.6% of patients and was due predominantly to strains with PTs other than those found in tap water and in the stomach. Moreover, when we analyzed only patients with VAP caused by PA, no strain was found previously in the stomach. These results agree with a study on the pathogenesis of VAP [21] and demonstrate that a gastric origin is unlikely in strains of PA causing VAP.
This study is also unique in subculturing at least four colonies that were representative of the different morphological types of PA present on each culture plate. It allowed the origin of the strains to be identified and reduced the risk of underestimating exogenous colonization. Unlike environmental strains, isolates from airways were mainly monoclonal. The implications for diagnosis and therapy in clinical practice are evident.
One particularly interesting finding was that strains present in tap water or in the environment were associated with colonization, but only infrequently with infection. Indeed, our results show that more than 70% of strains causing gastric or respiratory colonization come from tap water or environmental surfaces of the ICU, whereas among strains causing pulmonary infection, 50% were of endogenous or primary origin, and only one of the remaining strains come from tap water. Further studies analyzing exoproducts or different virulence proteins secreted by different strains of PA may help to explain these findings. In fact, recent studies [22, 23] have reported that isolates of PA able to secret type III proteins are of increased virulence, and pulmonary infections caused by these strains are associated with higher mortality or recurrence. Further studies should investigate whether intubated patients colonized with type III secreting isolates are at a higher risk of developing pneumonia than patients colonized by nonsecretory strains of PA.
Our findings suggest that efforts to prevent colonization by PA should be directed toward decontamination of tap water and infection control measures that reduce cross-contamination from exogenous sources. However, recent reports [17, 20] have noted how difficult it is to reduce colonization in tap water. Cross-contamination from exogenous origin was involved in one-half of our episodes of pneumonia, and our findings support the use of alcoholic solutions for hand antisepsis [24]. In fact, our findings changed the infection control strategies in our ICU: only mineral water to administer drugs through the gastric tube, and alcohol solution was used before contact with a new patient.
Important limitations of our study were that not all intubated patients were consecutively included, and that samples for culture were obtained each 72 h. Cross-contamination from other colonized patients could thus not be detected, and in the cases with simultaneous detection of PA at different sites the true sequence of colonization remains uncertain. Other studies applying similar methods for sample collection (twice weekly) have had the same problem of interpretation [25]. Another limitation is the that only a reduced number of surveillance cultures of the environmental surfaces and hands of HCW were carried out. Although the incidence rate of hands colonized by PA was similar to that in a study [26] carried out in a neonatal ICU with endemic PA infection, increasing the number of cultures from environment and HCW should allow us to determine more exactly the importance of cross-colonization. Finally, our findings should not be generalized to ICUs with different colonization pressures and case-mixes.
In summary, although the origin of PA colonization in intubated patients is often exogenous, in patients who developed pulmonary infection the origin can be either exogenous or endogenous. Our results confirm that the ICU environment is a major source of micro-organisms colonizing critically ill patients and in particular emphasize the importance of exogenous colonization/infection of PA. A combination of early identification (and eradication) of airways colonization by PA plus infection control measures targeted to reduce cross-contamination should be the basis to prevent pulmonary infection.
References
Rello J, Rué M, Jubert P, Muses G, Soñora R, Vallés J, Niederman MS (1997) Survival in patients with nosocomial pneumonia: impact of the severity of illness and the etiologic agent. Crit Care Med 25:1862–1867
Brewer SC, Wunderink RG, Jones CB, Leeper KV (1996) Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 109:1019–1029
National Nosocomial Infections Surveillance System (1999) Data summary from January 1990–May 1999, issued June 1999. Am J Infect Control 27:520–532
Talon D, Mulin B, Rouget C, Bailly P, Thouverez M, Viel JF (1998) Risks and routes for ventilator-associated pneumonia with Pseudomonas aeruginosa. Am J Respir Crit Care Med 157:978–984
Bergmans DC, Bonten MJ, van Tiel FH, Gaillard CA, van der GS, Wilting RM, de Leeuw PW, Stobberingh EE (1998) Cross-colonisation with Pseudomonas aeruginosa of patients in an intensive care unit. Thorax 53:1053–1058
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Chastre J, Fagon JY, Trouillet JL (1995) Diagnosis and treatment of nosocomial pneumonia in patients in intensive care units. Clin Infect Dis 21 [Suppl 3]:S226–S237
El-Ebiary M, Torres A, González J, Puig de la Bellacasa J, García C, Jiménez de Anta MT, Ferrer M, Rodríguez-Roisin R (1993) Quantitative cultures of endotracheal aspirates for the diagnosis of ventilator-associated pneumonia. Am Rev Respir Dis 148:1552–1557
Gilchrist MJR (1992) Microbiological culturing of environmental and medical-device surfaces. In: Isenberg HD (ed) Clinical microbiology procedures handbook. American Society for Microbiology, Washington
Cortés P, Mariscal D, Vallés J, Rello J, Coll P (2001) Implications of the presence of polyclonal Pseudomonas aeruginosa in an intensive care unit. A 27-month prospective study on molecular epidemiology. Infect Control Hosp Epidemiol 22:720–723
Tenover FC, Arbeit RD, Goring RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239
Döring G, Hörz M, Ortelt J, Grupp H, Wolz C (1995) Molecular epidemiology of Pseudomonas aeruginosa in an intensive care unit. Epidemiol Infect 110:427–436
Olson B, Weinstein RA, Nathan C, Chamberlin W, Kabins SA (1984) Epidemiology of endemic Pseudomonas aeruginosa: why infection control efforts have failed. J Infect Dis 150:808–816
Speijer H, Savelkoul PH, Bonten MJ, Stobberingh EE, Tjhie JH (1999) Application of different genotyping methods for Pseudomonas aeruginosa in a setting of endemicity in an intensive care unit. J Clin Microbiol 37:3654–3661
Bertrand X, Thouverez M, Talon D, Boillot A, Capellier G, Floriot C, Helias JP (2001) Endemicity, molecular diversity and colonisation routes of Pseudomonas aeruginosa in intensive care units. Intensive Care Med 27:1263–1268
Bonten MJ, Bergmans DC, Speijer H, Stobberingh EE (1999) Characteristics of polyclonal endemicity of Pseudomonas aeruginosa colonization in intensive care units. Implications for infection control. Am J Respir Crit Care Med 160:1212–1219
Trautmann M, Michalsky T, Wiedeck H, Radosavljevic V, Ruhnke M (2001) Tap water colonization with Pseudomonas aeruginosa in a surgical intensive care unit (ICU) and relation to Pseudomonas infections of ICU patients. Infect Control Hosp Epidemiol 22:49–52
Bert F, Maubec E, Bruneau B, Berry P, Lambert-Zechovsky N (1998) Multi-resistant Pseudomonas aeruginosa outbreak associated with contaminated tap water in a neurosurgery intensive care unit. J Hosp Infect 39:53–62
Ferroni A, Nguyen L, Pron B, Quesne G, Brusset MC, Berche P (1998) Outbreak of nosocomial urinary tract infections due to Pseudomonas aeruginosa in a pediatric surgical unit associated with ta-water contamination. J Hosp Infect 39:301–307
Reuter S, Sigge A, Wiedeck H, Trautmann M (2002) Analysis of transmission pathways of Pseudomonas aeruginosa between patients and tap water outlets. Crit Care Med 30:2222–2228
Bonten MJ, Gaillard CA, van Tiel FH, Smeets HGW, van der Geest S, Stobberingh EE (1994) The stomach is not a source for colonization of the upper respiratory tract and pneumonia in ICU patients. Chest 105:878–884
Hauser AR, Cobb E, Bodí M, Mariscal D, Vallés J, Engel JN, Rello J (2002) Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med 30:521–528
Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP (2001) Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis 183:1767–1774
Pittet D (2001) Compliance with hand disinfection and its impact on hospital-acquired infections. J Hosp Infect 48 [Suppl A]:S40–S46
Feldman C, Kassel M, Cantrell J, Kaka S, Morar R, Goolam MA, Philips JI (1999) The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur Respir J 13:546–551
Foca M, Jakob K, Whittier S, Della LP, Factor S, Rubenstein D, Saiman L (2000) Endemic Pseudomonas aeruginosa infection in a neonatal intensive care unit. N Engl J Med 343:695–700
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by a grant from the FIS 96/146
Rights and permissions
About this article
Cite this article
Vallés, J., Mariscal, D., Cortés, P. et al. Patterns of colonization by Pseudomonas aeruginosa in intubated patients: a 3-year prospective study of 1,607 isolates using pulsed-field gel electrophoresis with implications for prevention of ventilator-associated pneumonia. Intensive Care Med 30, 1768–1775 (2004). https://doi.org/10.1007/s00134-004-2382-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2382-6