Abstract
Objective
To evaluate the ability of the new, built-in occlusion pressure (P0.1) measurement to predict extubation failure.
Design and setting
Prospective observational multicentre study in the ICU of five general hospitals.
Patients
Hundred thirty patients on mechanical ventilation longer than 48 h when considered ready for weaning.
Measurements and results
Patients underwent a 30-min spontaneous breathing trial with simultaneous monitoring of occlusion pressure (P0.1) and breathing pattern (f/Vt). Sixteen patients (12%) failed the weaning trial and full ventilatory support was resumed, while 114 tolerated the trial and were extubated. Twenty-one (18%) required reintubation within 48 h. The area under the ROC curve for diagnosing extubation failure was 0.53 for f/Vt, 0.59 for P0.1 and 0.61 for P0.1*f/Vt (p=NS). Accordingly, P0.1*f/Vt more than 100 detected extubation failure with a sensitivity of 0.89, specificity of 0.35, positive predictive value of 0.21 and negative predictive value of 0.94.
Conclusion
During a first trial of spontaneous breathing on pressure support ventilation (PSV), bedside P0.1 and P0.1*f/Vt are of little help, if any, for predicting extubation failure.
Similar content being viewed by others
Introduction
Liberation from mechanical ventilation still consumes substantial medical resources. Recent randomised clinical trials [1] focusing on the early detection of ready-to-wean patients have demonstrated that this approach may reduce ventilator days, ICU length of stay and probably outcome. Based on these studies, clinical protocols are increasingly being implemented in intensive care departments [2]. Weaning failure is usually defined as the development of significant distress when ventilatory support is withdrawn, or as the need for reintubation within a fixed period of time after extubation [3, 4, 5]. However, only reintubation is clearly associated with a poor prognosis. Interestingly, during the spontaneous breathing trial patients needing reintubation commonly seem indistinguishable from successfully weaned patients [4] and extubation failure prediction is, as yet, not available at the bedside. Nevertheless, even in the most recent clinical trials, the mean reintubation ratio ranges between 12 and 25% [3, 4, 5].
The fact that extubation failure is associated with morbidity and mortality is of great relevance [5]. Some recent analyses suggest that reintubation may be an independent predictor of ICU mortality after adjusting for severity of illness scores [6]. Accordingly, extensive research has been carried out to investigate the usefulness of various parameters as predictors of extubation failure [7, 8, 9, 10]. From a clinical point of view, only the “rapid shallow breathing index” (f/Vt) [11] is generally accepted. With the intrinsic dependence on technical expertise and sophisticated equipment, routine use of some of the other parameters suggested has never reached wide acceptance. One such parameter, the pressure in the first 100 ms of an occluded inspiration (P0.1), was studied during weaning as an isolated predictor [12, 13], but was later linked with maximal inspiratory pressure (PIMax) [14, 15], hypercapnic challenge [16] and f/Vt [17].
Measurement of occlusion pressure has recently become clinically available at the bedside and has been incorporated in some ventilators [18, 19, 20]. The clinical usefulness of this new technology in diagnosing extubation failure must therefore be evaluated. Knowing in advance that conditions masked by intubation (upper airway resistance, laryngospasm, aspiration, etc.) will not be detected by any “respiratory drive test”, we attempted to find a way to detect a subgroup of patients with subclinical respiratory troubles while still intubated. Our objective was to test the diagnostic power of the now clinically available P0.1 system to predict extubation failure, instead of spontaneous breathing trial failure. First, the threshold values of the indices that best discriminated between successful weaning and extubation failure were determined in half the population; second, the accuracy of each index was then assessed in the remaining half. Data thus obtained provided information on the time course of f/Vt, P0.1 and P0.1-derived parameters during a 30-min spontaneous breathing test.
Material and methods
Patients
In five medical-surgical ICUs, a total of 130 critically ill patients over 18 years of age on mechanical ventilation for more than 48 h were studied until a first attempt was made to discontinue ventilator support. To be enrolled in the study, patients had to have an improvement or resolution of the underlying cause of respiratory failure; adequate gas exchange, as indicated by a PaO2 more than 60 mmHg at FIO2 of 0.4 or less with a PEEP 5 cmH2O or less; a Glasgow Coma Scale score above 13; a core temperature below 38°C; a haemoglobin level above 8 g/dl and no further need for vasoactive or sedative agents. As our study was focused to predict extubation failure taking into account the two aspects, the tolerance of ventilatory support withdrawal and withdrawal of the artificial airway, tracheostomised patients were excluded. Lack of available ventilators equipped with P0.1 measurement was the only additional exclusion criteria.
The ethics committees at the participating hospitals approved the study and informed consent was waived because of the non-interventional nature of the trial.
Protocol
When a patient was enrolled in the study, spontaneous breathing ability was checked by switching the ventilator (Evita 2 and Evita 4, Draeger, Lübeck, Germany) from full ventilatory support to CPAP 5 cmH2O for 3 min. The flow trigger was set at 1 l/min. Tidal volume (Vt), respiratory rate (RR) and P0.1 were recorded from the ventilator display, whereas f/Vt was calculated. When RR was less than 35 breaths/min and Vt was more than 5 ml/kg, patients underwent a trial of spontaneous breathing. Patients who did not meet these criteria when first tested were re-evaluated on a daily basis.
The spontaneous breathing trial consisted of a 30-min period of 7 cmH2O-pressure support ventilation (PSV) with zero PEEP in the ventilator [7]. The flow trigger was set at 1 l/min. Other approaches that may influence the breathing pattern, such as the automatic tube compensation, were not used. Heart rate (HR), systolic blood pressure (SBP), oxygen saturation (SaO2) measured by pulse oximetry, RR and occlusion pressure (P0.1) were recorded every 10 min. If the patient had any of the following signs of poor tolerance: RR 35 or more, SaO2 less than 90%, HR higher than 140 or a sustained change in HR greater than 20%, SBP 200 mmHg or more or less than 80 mmHg, and agitation, diaphoresis or anxiety, the attending physician stopped the trial and re-instituted mechanical ventilation. Patients who showed no signs of poor tolerance at the end of the trial were immediately extubated and supplemental oxygen was administered by face-mask.
Extubation failure was defined as the need for reintubation within 48 h after extubation. To avoid confounding factors, non-invasive mechanical ventilation was not allowed in the 48-h post-extubation period. Patients were followed up until hospital discharge or death.
Procedures
Occlusion pressure (P0.1) was measured by means of the built-in system of the ventilator Draeger Evita, widely described elsewhere [18, 19]. The reported systematic difference between Evita and standard P0.1 measurement is 0.3±0.5 cmH2O in a test model and 0.6±0.7 cmH2O in patients [18]. We averaged five P0.1 measurements as the P0.1 value at each point of the study: baseline, 10, 20 and 30 min during the spontaneous breathing trial with PSV.
Data and statistical analysis
Based on previous experiences [3, 4, 5], the expected spontaneous breathing trial success ranged between 85 and 90%, and reintubation rates ranged from 10 to 25%, with an estimated sample size of 105 patients.
Because of its non-normal distribution, data are presented as median and 25th and 75th percentiles. Categorical variables were analysed with Fisher’s exact tests. Kruskal-Wallis analysis was used to compare continuous variables among patients who failed the spontaneous breathing trial, those who needed reintubation and those who were successfully extubated. Because our target was extubation failure, a “true positive” was defined as a patient who failed extubation and showed a positive test (i.e. P0.1 greater than the cut-off), whereas a “true negative” was a successfully extubated patient who showed a negative test (i.e. P0.1 lower than the cut-off). Sensitivity, specificity, positive predictive value and negative predictive value were calculated. Because sensitivity and specificity are highly dependent on the total number of patients studied, the predictive power of each index to detect extubation failure was assessed by likelihood ratios, both of a positive test and of a negative test. Furthermore, the predictive performance of increments in each index was evaluated using receiver operating characteristic (ROC) curves, an analysis that is not dependent on the threshold value selected.
Results
Of 130 patients included in the study, 16 (12%) failed the spontaneous breathing trial, while 114 (88%) tolerated the trial and were immediately extubated. Of these, 21 (18%) required reintubation within 48 h. Clinical reasons for reintubation were: stridor in two cases, severe hypoxaemia in six, hypercapnia (ΔPaCO2 >15 mmHg) in three, extenuating work of breathing (paradoxical abdominal motion) in three, mucus plugging in four, cardiogenic lung oedema in one and lethargy in two. Ten patients were reintubated within 24 h, and 11 between 24 and 48 h.
Clinical characteristics in the three groups of patients are shown in Table 1. The only difference was the longer duration of ventilatory support before the spontaneous breathing trial in patients who failed the trial, as compared to patients who failed extubation. Table 2 summarises the functional indices in the first minute of spontaneous breathing in each group.
Outcome was clearly different depending on the results of the weaning trial (Table 3). Successfully extubated patients needed shorter ICU stays (median 11 days vs 20 days in extubation failure patients and 18 days in trial failure, p<0.05) and hospital stays (median 26 days vs 35 days in extubation failure and 34 days in trial failure, p<0.05). Mortality was greater in the extubation failure group compared with successfully extubated patients and with the trial failure group.
Patients who tolerated the spontaneous breathing trial were assigned to one of two data sets, a “training set” or a “prospective-validation set”, depending on the order in which they entered the study. The “training set” consisted of the first 57 patients, 45 of whom were successfully extubated while 12 needed reintubation. Data on these patients were used to determine which value for each index studied best differentiated between patients who were successfully extubated and those in whom extubation failed. The threshold value was taken as that which resulted in the best negative predictive value with a clinically significant sensitivity. This decision was based on the assumption that the disadvantages associated with extubation failure were higher than those derived from misclassification of a patient as “at risk of extubation failure”. The predictive power of the threshold value for each index was assessed in the remaining 57 patients, who made up the “prospective-validation” set, 48 of whom were successfully extubated while 9 failed extubation. The extubation failure rate was not different between the two groups.
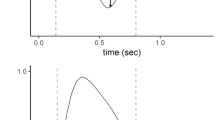
The ROC curve of each index in the total population of patients who tolerated the breathing trial is shown in Fig. 1. For the three indices, the areas were slightly higher than that of an arbitrary test that was expected a priori to have no discriminatory value (i.e. 0.50). During the first minute of spontaneous breathing, the threshold values for each index that best discriminated in the training set of data between successfully extubated patients and patients in whom reintubation was needed were 50 breaths/min per l for f/Vt, 2.8 cmH2O for P0.1 and 100 cmH2O*breaths/min per l for P0.1*f/Vt.
The accuracy of each index was tested in the prospective-validation data set as shown in Table 4. The main results showed that the best positive and negative predictive values were for the index P0.1*f/Vt with PPV of 0.21 and NPV of 0.94. In accordance with the design of the study, the likelihood ratio of a negative test was higher, while the likelihood ratio of a positive test was marginal.
Figure 2 shows the time-course of the three indices during the spontaneous breathing trial. The extubation failure group and the successful extubation group showed no significant differences throughout the 30-min period of spontaneous breathing, whereas a slight tendency to increase (<15%) was observed in P0.1 and P0.1*f/Vt in patients who needed reintubation.
Discussion
The results from the present study may help to detect those patients with a higher likelihood of failing extubation after a successful test of spontaneous breathing. Whether such a diagnosis means that a patient requires a longer period of intubation or whether his breathing should be assisted using other means (i.e. non-invasive mechanical ventilation) has yet to be elucidated.
A collective Task Force recently developed evidence-based guidelines for weaning and discontinuing ventilatory support [7]. This Task Force stated that “...assessment techniques to identify patients who are capable of ventilator discontinuation need to be utilized. Ideal assessment techniques should be able to easily and safely distinguish which patients need prompt discontinuation and which need continued ventilatory support”. The most recent trials [1, 3, 4, 5] have revealed that up to 20–40% of patients screened to discontinue mechanical ventilation are unable to sustain spontaneous breathing, either because of intolerance to the test or because of the need for reintubation. While half are correctly classified as they present intolerance, the other half tolerate tube withdrawal but develop respiratory difficulties. This latter population is thus exposed to the risks of extubation failure without any kind of warning for physicians. Additionally, the high morbidity and mortality associated with reintubation emphasises the need for tools to diagnose extubation failure.
The present multicentre trial confirms the proportion of patients who do not tolerate the breathing trial and patients who fail extubation after a correct breathing trial. Accordingly, our outcomes also appear very similar to those of previous trials [3, 4, 5], showing again the poor prognosis of patients who need reintubation.
Our cut-off points, using a mathematical approach, were lower than those previously reported [11, 17]. It should be noted that this difference is highly related to patient selection and, in part, to the use of PSV. In contrast with other studies, our inclusion criteria, mainly respiratory rate and tidal volume, virtually eliminate patients with high f/Vt ratios. Indeed, only 2 out of 114 patients showed a f/Vt ratio greater than 100. When looking at P0.1 and P0.1*f/Vt values, weaning failure rates were also lower than the values previously reported [15, 17] because the patients with higher respiratory centre activity commonly showed rapid shallow breathing and were also excluded. In other words, our trial deals with patients who are very difficult to assess in terms of extubation failure.
The lower threshold values observed for discriminating extubation failure may also be the result of the fact that patients breathed with some ventilatory support. Whether our low-level pressure support ventilation aided the fully spontaneous breathing or only compensated for the burden of ventilator valves, circuitry and additional dead space remains unknown. The P0.1 decrease is proportional to the PSV increase as long as the PSV unloads the respiratory muscles [21]. In the Alberti et al. study [22], ventilator-dependent patients with acute respiratory failure showed a P0.1 value of 0.8±0.5 cmH2O when fully supported with PSV and P0.1 of 4.2±2.7 cmH2O when PSV was reduced to 50% of the initial value. In the present study, we used a low level of PSV during the breathing trial, equal to that reported by Esteban et al. [5] who found no changes in clinical outcome in comparison with T-tube during the trial. These data reinforce the idea that this low-level PSV only compensates for the additional work imposed by the ventilator and the circuitry.
Our values of baseline P0.1 in both groups correlated with those of the Hilbert et al. study [23] in COPD patients. These authors found pre-extubation P0.1 values of 2.4±0.9 and 2.9±0.7 cmH2O in successfully and unsuccessfully weaned COPD patients, respectively. The lack of statistical difference may be due to the small sample size. Moreover, they found a non-significant tendency to higher P0.1*f/Vt values in extubation failure (206±115 vs 158±110 cmH2O*breaths/min per l). The fact that they studied a selected population of COPD patients with a high extubation failure ratio (32%) precludes the direct extrapolation of the data to the whole weaning ICU population at risk.
The reason for having a time period of spontaneous breathing before extubation is to try to ascertain whether patients will be able to sustain breathing in the long term [7]. While this seems to be valid for patients who do not tolerate the test, it does not detect those patients who will need reintubation after tolerating the test. Previous studies have shown that common clinical parameters, mainly respiratory rate, tidal volume and heart rate, are also unreliable in predicting extubation failure. In the study by Esteban et al. [4] these parameters showed no changes at all from the beginning to the end of the breathing trial, neither in successfully extubated patients nor in those who failed extubation. The present study is also original in showing results on the time course of P0.1 and P0.1-derived parameters during the 30-min spontaneous breathing trial. In our successfully extubated patients, P0.1 and derived parameters were stable throughout the spontaneous breathing trial, but showed a non-significant trend to increase in patients who ultimately needed reintubation.
Nevertheless, the magnitude of the P0.1 and P0.1*f/Vt increase was about 15% and appears to be within the coefficient of variation for the measurement. In models with a significant effect of the mechanical ventilator on the level of ventilatory support, P0.1 has been closely correlated with patients’ work of breathing [22]. In fully supported ventilated COPD patients, Mancebo et al. found that P0.1 was a sensitive marker of the PEEP effect to counterbalance autoPEEP [24] with its associated reduction in work of breathing. The possibility of an artefact of autoPEEP in our P0.1 values was negligible because PEEP was not used at any time during the PSV breathing trial.
Therefore, taking into account that P0.1 is a sensitive marker of respiratory centre stimulus [25, 26, 27, 28], the fact that only a minority of our unsuccessful patients showed an increase in P0.1 with the increase in respiratory effort induced by the breathing trial suggests that the factors responsible for extubation failure were not present before extubation or were not related to impending respiratory failure. In either case, it would seem that prediction of extubation failure is unlikely to improve with presently available clinical respiratory parameters. Again, the lack of improvement in the diagnostic power of our P0.1 and P0.1-derived parameters after extending the time of observation throughout the breathing trial reinforces two previously reported issues: first, that most clinical data suggesting inability to sustain long-term spontaneous breathing are evident in the first few minutes after disconnecting the ventilatory support [11] and, second, that the optimal duration of the breathing trial remains controversial but could probably be reduced to less than 30 min [4].
In conclusion, even when clinical selection is made with great care and a 30-min trial of spontaneous breathing is performed, as many as 18% of patients require reintubation. In this group of life-threatened patients, measuring P0.1 and P0.1*f/Vt on low PSV during the breathing trial is of little help, if any, for predicting extubation failure.
References
Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, Johnson MM, Browder RW, Bowton DL, Haponik EF (1996) Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 335:1864–1869
Saura P, Blanch Ll, Lucangelo U, Fernandez R, Mestre J, Artigas A (1996) Utility of capnography to detect hypercapnic episodes during weaning from mechanical ventilation. Intensive Care Med 22:374–381
Esteban A, Frutos F, Tobin MJ, Alía I, Solsona JF, Valverdú I, Fernandez R, de la Cal MA, Benito S, Tomas R, Carriedo D, Macias S, Blanco J (1995) A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med 332:345–350
Esteban A, Alia I, Tobin MJ, Gil A, Gordo F, Vallverdu I, Blanch Ll, Bonet A, Vazquez A, de Pablo R, Torres A, de la Cal MA, Macias S (1999) Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Am J Respir Crit Care Med 159:512–518
Esteban A, Alia I, Gordo F, Fernandez R, Solsona J, Vallverdu I, Macias S, Allegue J, Blanco J, Carriedo D, Leon M, de la Cal M, Taboada F, Velasco J, Palazon E, Carrizosa F, Tomas R, Suarez J, Goldwasser R (1997) Extubation outcome after spontaneous breathing trials with t-tube or pressure support ventilation. Am J Respir Crit Care Med 156:459–465
Epstein SK, Ciubotaru RL, Wong JB (1997) Effect of failed extubation on the outcome of mechanical ventilation. Chest 112:186–192
MacIntyre NR, Cook DJ, Ely EW Jr, Epstein SK, Fink JB, Heffner JE, Hess D, Hubmayr RD, Scheinhorn DJ (2001) Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 120 (6 Suppl): 375S–395S
Saura P, Blanch Ll, Mestre J, Vallés J, Artigas A, Fernandez R (1996) Clinical consequences of the implementation of a weaning protocol. Intensive Care Med 22:1052–1056
Vallverdu I, Calaf N, Subirana M, Net A, Benito S, Mancebo J (1998) Clinical characteristics, respiratory functional parameters and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med 158:1855–1862
Yang KL (1993) Inspiratory pressure/maximal inspiratory pressure ratio: a predictive index of weaning outcome. Intensive Care Med 19:204–208
Yang KL, Tobin MJ (1991) A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 324:1445–1450
Sassoon CS, Te TT, Mahutte CK, Light RW (1987) Airway occlusion pressure. An important indicator for successful weaning in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 135:107-113
Sassoon CS, Mahutte CK, Te TT, Simmons DH, Light RW (1988) Work of breathing and airway occlusion pressure during assist-mode mechanical ventilation. Chest 93:571-576
Fernandez R, Cabrera J, Calaf N, Benito S (1990) P0.1/PIMax: an index for assessing respiratory capacity in acute respiratory failure. Intensive Care Med 16:175-179
Gandia F, Blanco J (1992) Evaluation of indexes predicting the outcome of ventilator weaning and value of adding supplemental inspiratory load. Intensive Care Med 18:327–333
Montgomery A, Holle R, Neagley S, Pierson D, Schoene R. (1987) Prediction of successful ventilator weaning using airway occlusion pressure and hypercapnic challenge. Chest 91:496-499
Sassoon CS, Mahutte CK (1993) Airway occlusion pressure and breathing pattern as predictors of weaning outcome. Am Rev Respir Dis 148:860–866
Kuhlen R, Hausmann S, Pappert D, Slama K, Rossaint R, Falke K (1995) A new method for P0.1 measurement using standard respiratory equipment. Intensive Care Med 21:554–560
Subirana M, Irrazabal C, Bak E, Jara F, Mancebo J (1997) Evaluacion de la medida de la presion de oclusion incorporada en los ventiladores Evita. Med Intensiva 21:305–310
Fernandez R, Benito S, Sanchis J, Milic-Emili J, Net A (1988) Inspiratory effort and occlusion pressure in triggered mechanical ventilation. Intensive Care Med 14:650-653
Fernandez R (2001) Timing and criteria for beginning weaning. In: Mancebo J, Net A, Brochard L (eds) Mechanical ventilation and weaning. Springer, Berlin, pp 239–247
Alberti A, Gallo F, Fongaro A, Valenti S, Rossi A (1995) P0.1 is a useful parameter in setting the level of pressure support ventilation. Intensive Care Med 21:547–553
Hilbert G, Gruson D, Portel L, Vargas F, Gbikpi-Benissan G, Cardinaud JP (1998) Airway occlusion pressure at 0.1 s (P0.1) after extubation: an early indicator of postextubation hypercapnic respiratory insufficiency. Intensive Care Med 24:1277–1282
Mancebo J, Albaladejo P, Touchard D, Bak E, Subirana M, Lemaire F, Harf A, Brochard L (2000) Airway occlusion pressure to titrate positive end-expiratory pressure in patients with dynamic hyperinflation. Anesthesiology 93:81–90
Whitelaw W, Derenne JP, Milic-Emili J (1975) Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol 23:181-199
Murciano D, Boczkowski J, Lecocguic Y, Milic-Emili J, Pariente R, Aubier M (1988) Tracheal occlusion pressure: a simple index to monitor respiratory muscle fatigue during acute respiratory failure in patients with chronic obstructive pulmonary disease. Ann Intern Med 108:800-805
Dimarco A, Kelsen S, Cherniack N, Gothe B (1983) Occlusion pressure and breathing pattern in patients with interstitial lung disease. Am Rev Respir Dis 127:425-430
Herrera M, Blasco J, Venegas J, Barba R, Doblas A, Marquez E (1985) Mouth occlusion pressure (P0.1) in acute respiratory failure. Intensive Care Med 11:134-139
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernandez, R., Raurich, J.M., Mut, T. et al. Extubation failure: diagnostic value of occlusion pressure (P0.1) and P0.1-derived parameters. Intensive Care Med 30, 234–240 (2004). https://doi.org/10.1007/s00134-003-2070-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-2070-y