Abstract
Objective: To evaluate the effect of four doses of intravenous glutamine supplementation on skeletal muscle metabolism. Design: A prospective, blinded, randomized study. Setting: The general Intensive Care Unit (ICU) of a university hospital. Patients: ICU patients with multiple organ failure (n=40), who were expected to stay in the unit for more than five days. Intervention: Patients received 0, 0.28, 0.57 or 0.86 g of glutamine per kg bodyweight per day intravenously for five days as part of an isocaloric, isonitrogenous and isovolumetric diet. Results: Plasma glutamine concentration responded to glutamine supplementation with normalization of plasma levels in a dose-dependent way, while free muscle glutamine concentration, as well as muscle protein synthesis and muscle protein content, did not change significantly. Conclusion: Intravenous glutamine supplementation to ICU patients for a period of five days resulted in normalization of plasma glutamine concentrations in a dose-dependent way whereas muscle glutamine concentrations were unaffected.
Similar content being viewed by others
Introduction
The effects of glutamine supplementation on clinical outcome have been summarized in a recent meta-analysis [1]. The morbidity and mortality of intensive care patients is reduced [2–5], as well as the morbidity and length of hospital stay following major elective surgery [6, 7]. Although the clinical effects of glutamine depletion are preferably seen in intestinal organs and in the immune system, skeletal muscle holds a central role in glutamine metabolism as the main producer and exporter of glutamine. In Intensive Care Unit (ICU) patients, muscle free glutamine concentration is reduced by 75% early in the course of disease [8, 9]. It has been shown that glutamine production is enhanced in the critically ill at the expense of muscle proteins [10], but it is obviously not sufficient to maintain intracellular levels of glutamine in muscle. Exogenous glutamine supplementation has, therefore, been suggested to supply sufficient amounts of glutamine to target organs, and in parallel to prevent undesired muscle wasting.
Available reports indicate that glutamine-supplemented nutrition to ICU patients to be only marginally effective in restoring muscle protein synthesis rate and muscle glutamine content [11, 12]. However, the dose of glutamine given in these earlier studies was 0.28 g per kg body weight (bw) and day, which is insufficient to restore the concentration of glutamine in plasma and in muscle. Furthermore, there are pilot studies indicating that a higher dose of glutamine may have an effect on muscle glutamine concentration [13].
The aim of the present study was to investigate three different levels of glutamine supplementation to critically ill patients. The effects on muscle were primarily evaluated in terms of muscle glutamine concentration, muscle protein synthesis and muscle protein content. In addition, the concentrations of DNA, RNA, energy-rich phosphates, water, electrolytes, lactate and glutathione were studied, as well as urinary excretion of nitrogen and 3-methylhistidine.
Materials and methods
Subjects and experimental protocol
Critically ill patients (n=4×10) submitted to the multi-disciplinary Intensive Care Unit (ICU) at Huddinge University Hospital between October 1997 and February 1999, who were expected to stay for more than five days were included in the study. Exclusion criteria were children (below 18 years), pregnancy, patients with severe liver failure, patients undergoing dialysis and patients with coagulation disorders precluding muscle biopsies. Prior to hospital admission, all patients lived in their own homes, had full-time work, if not retired due to age (over 65 years). Patient characteristics are given in Table 1.
Parenteral nutrition was given continuously throughout the study, with a change of containers at 14:00 each day according to the routine of the unit. Patient care and treatment were in every other aspect in accordance with the routine of the unit. All patients except one (no. 8) were intubated and mechanically ventilated at the beginning of the study. If propofol was given as sedation, the amount of fat emulsion given as nutrition was consequently and continuously adjusted. In six cases analgesia was given via an indwelling epidural catheter. At the end of the study period, seven out of 39 patients were weaned off the ventilator.
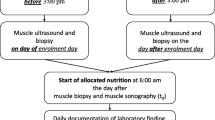
Patients were studied twice: once before and once at the end of a five-day intervention period. The patients were randomized to receive total parenteral nutrition (TPN) without glutamine supplementation or TPN supplemented with glutamine at a dose of 0.28, 0.57 or 0.86 g of glutamine/kg bw/day, corresponding to 0, 20, 40 or 60 g of glutamine per day for a 70 kg man, given as a continuous infusion over 24 h.
Randomization was performed by the closed envelope system after inclusion. Blood and tissue samples were blinded throughout the procedure for sample analysis, but treatment was not blinded for attending physicians.
The five-day study period started on average on day three (range 1–26) of ICU stay. The first determination of protein synthesis rate was performed on day one of the study. An injection of L-[2H5] phenylalanine (45 mg/kg bw as a 2% solution, 15 atom%excess) was given intravenously over 10 min. A muscle biopsy was taken before and 90 min after the isotope injection. The procedure to obtain percutaneous muscle biopsies from the lateral portion of the quadriceps femoris muscle has been described in detail previously [14].
Arterial blood samples were taken from the radial artery every morning during the study period for plasma amino acid determinations. Urine was collected in 24-h portions from between 06:00 to 06:00 from day one, 8 h before the start of glutamine supplementation until the morning of day seven, 16 h after the end of the study period.
The patients, or if communication was not possible, the relatives of the patient, were informed of the nature, purpose, procedures and potential risks involved in the study before obtaining their voluntary consent to participate in the study. The study protocol was approved by the Ethical Committee at Karolinska Institutet, Huddinge University Hospital, Stockholm, Sweden.
Parenteral nutrition
Prior to the study no glutamine-containing intravenous feeding was given to the patients. During the study period all patients received parenteral nutrition with a nonprotein energy content of 126 kJ/kg bw/24 h. The nonprotein calories were provided as equal amounts of glucose (200 mg/ml; B. Braun Medical) and fat (Intralipid, 200 mg/ml; Fresenius Kabi). In the control group the nitrogen was given as 0.18 g N/kg bw/24 h (Vamin, 18 g N/l; Fresenius Kabi). In the groups receiving total parenteral nutrition supplemented with glutamine, the glutamine was provided as Glavamin (Fresenius Kabi) combined with a solution containing L-glutamine (36 mg/ml in a glucose solution of 100 mg/ml). Nutrition was isocaloric and isonitrogenous for all groups and infusion was isovolumetric (36–43 ml/kg bw/24 h). The glucose solution containing L-glutamine was prepared by the hospital Pharmacy Department. The total parenteral nutrition was given continuously from 14:00 to 14:00 according to the routines of the unit.
Analyses
The measurement of protein synthesis rate in muscle tissue of man by the flooding dose technique employing gas chromatography-mass spectrometry has been described in detail previously [15].
The free amino acids in plasma and skeletal muscle were determined by ion exchange chromatography after derivatization with o-phtaldialdehyde as described previously [16].
Thiols in human muscle tissue were determined by HPLC, as described by Luo et al. [17].
The assays for water, electrolyte, protein, nucleic acids, lactate and energy-rich phosphates have been described in detail elsewhere [18].
The total nitrogen content of the urine was determined by a chemiluminescent nitrogen system (771C Pyroreactor, 720C Nitrogen Detector; ANTEK, Houston, Texas, USA), and 3-methylhistidine in urine was analyzed using HPLC [19].
Calculations and statistics
All data are presented as means±SD if not indicated otherwise. The statistical analysis was performed with analysis of variance ANOVA/MANOVA for repeated measure employing Statistica for Windows (StatSoft, Tulsa, USA). Correlations were evaluated using Pearson’s linear correlation coefficient.
Results
Thirty-seven out of 40 randomized critically ill patients were able to adhere to the full protocol for the five-day study period, with determination of muscle protein synthesis rate, muscle free amino acids and other muscle parameters at two occasions, day one and day six, blood sampling every day and urine collection in 24-h portions during the whole study period. Three patients were excluded from the protocol; patient number 4 died on study day three, patient number 27 was rapidly weaned off the ventilator and transferred to another ward where it was not possible to continue the study, and patient number 40 completed the protocol but the second determination of muscle protein synthesis rate was not performed because the muscle biopsy material was lost. No statistically significant differences were observed between the groups in age, BMI, starting day, Apache II score or SOFA scores (Table 1).
Plasma amino acids
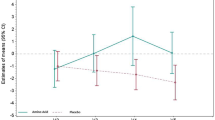
Total amino acids in plasma did not differ between the groups (Table 2). Glutamine concentration taken before intervention on day three (1–26) of ICU stay, was 376±160 μmol/l. Control patients remained on the same level, while the glutamine-treated groups showed a 32±35%, 72±53% and 87±55% increase for 0.28, 0.57 or 0.86 g of glutamine/kg bw/24 h, respectively (P<0.01), reaching a plateau after 24 h of glutamine supplementation (Fig. 1).
Plasma glutamine concentration (μmol/l) in ICU patients (n=4×10) randomized to receive 0 (◆), 0.28 (□), 0.57 (▲) or 0.86 (○) gram glutamine per kg body weight per day intravenously during a five-day study period. For all glutamine-treated groups the plasma glutamine concentration increased (P<0.01) from the first day of treatment and onwards. Data are expressed as means±SD
Muscle amino acids
The mean concentration of muscle free glutamine in the initial biopsy before intervention was 3.5±1.9 mmol/g wet weight and at the second biopsy, after treatment it was 4.5±2.3 mmol/g wet weight, which was not significantly different among any of the four groups of patients (Table 3). The results of the other free amino acid concentrations in muscle are given in Table 4.
Muscle protein synthesis rate
The mean protein fractional synthesis rate (FSR) at the time of the first biopsy was 1.61±0.62% per day (n=40) and it did not change significantly in any of the four groups, so there were no differences between the groups irrespective of whether glutamine supplementation was given or not (Table 3).
Thiols in skeletal muscle
Muscle concentrations of reduced glutathione and total glutathione before and after the five-day study period are given in Table 5. In addition, the glutathione precursors γ-glutamyl-cysteine and cysteine in their total and reduced concentrations are included. No significant changes in the levels could be detected during the study period or related to glutamine supplementation.
Fat, water and electrolytes in skeletal muscle
The fat and total water content of the muscle related to fat free solid (FFS) did not differ between the groups or change over time (Table 6). Intracellular water content was the same in all groups and did not change significantly between the first and second biopsy, whereas extracellular water increased significantly in the groups receiving 0.28 and 0.57 g glutamine (P<0.05). Hence, there was a significant difference between the combined glutamine treated groups and the control group (P<0.01).
Muscle protein and nucleic acids
The protein concentration expressed as alkali soluble protein (ASP) per DNA decreased in all patients by 11% between the first and second biopsy, although the 0.28-g glutamine supplemented group remained unaltered, no significant differences between the groups were observed (Table 3). The capacity for protein synthesis expressed as RNA/DNA [20] also remained unchanged during the five-day study period (Table 7).
Muscle lactate
The ICU patients in this study had high lactate concentrations in muscle, 18.8±7.6 mmol/kg fat free solid (FFS). The control group increased over time by 64±87%, from 14.5±4.2 to 22.4±9.1 mmol/kg fat free solid (P<0.05). In contrast, the glutamine-treated groups did not change significantly; before and after treatment for the 0.28 g group 21.9±12.3 and 19.8±6.2 for the 0.57 g group 20.3±4.6 and 17.5±6.3, and for the 0.86 g group 17.4±5.4 and 18.7±11.6 mmol/kg FFS, respectively.
Energy-rich phosphates in muscle
Concentrations of ATP, total creatine, free creatine, as well as ATP related to total creatine did not show any significant differences between the groups or between the two biopsies taken five days apart (Table 8). Phosphocreatine and the phosphorylated fraction of phosphocreatine decreased significantly in the control group (P<0.05) between the two biopsies, but remained unchanged in the glutamine-treated groups.
Urine analyses
No significant difference was seen in the cumulative excretion of 3-methylhistidine or total nitrogen related to the different levels of glutamine supplementation (Fig. 2a, b).
Discussion
The main hypothesis in this study was that a large enough dose of exogenous glutamine supplementation would influence plasma glutamine concentration and secondarily muscle free glutamine concentration, muscle protein synthesis and muscle protein content. The results showed that whereas glutamine supplementation for five days can restore plasma glutamine levels in ICU patients (Fig. 1), this did not directly influence muscle glutamine levels, muscle protein synthesis rates and muscle protein content.
This study, randomizing ICU patients to four different levels of glutamine supplementation demonstrated an increased plasma glutamine level in all three treatment groups. Plasma glutamine levels have been reported to be consistently low in ICU patients and also to be related to outcome as an independent variable [8, 9, 21]. Glutamine supplementation of 0.28 g/kg bw/day restored mean plasma glutamine levels to normal after 24 h of infusion. The uppermost glutamine supplementation (0.86 g/kg bw/day) increased plasma glutamine concentrations to normal in all individual patients and most values were at upper normal or even supra-physiological levels. Despite these high levels of plasma glutamine concentration, muscle concentrations of free glutamine were not influenced, indicating that muscle glutamine concentration in these patients might be more related to the state of general inflammation than to glutamine depletion. Since muscle levels were not influenced by the glutamine supplementation, the absence of any changes in muscle protein synthesis was an expected finding. In particular, as the average rate of muscle protein synthesis in ICU patients is not very different from that in normal individuals [22, 23], in contrast to the decrease seen following surgery involving moderate stress [24].
The fact that plasma glutamine concentration can be restored into the normal range by exogenous glutamine supplementation, while the control group remained subnormal, is of course a feasible explanation to the positive clinical results reported when glutamine supplementation is given to ICU patients [1, 2, 4]. This also indicates that free glutamine status in muscle tissue may not be the crucial factor for immediate survival. Instead, availability of glutamine for other cells — preferably in the splanchnic region — may be most important during the ICU stay. However, to prove that the mortality rate for ICU patients decreases when their plasma glutamine concentration is normalized takes a prospective controlled clinical trial. Such a trial would require more than 2,000 patients, if a hypothesis of a decrease in mortality rate of 5% is to be proven or dismissed. From existing clinical studies there are clear indications that patients given glutamine supplementation for a longer period of time are those who benefit most [4]. A recent study shows no difference in clinical outcome after five days of treatment, while patients receiving more than nine days of treatment had a statistically significant better outcome [4]. At the time when the present study was designed a five-day treatment period seemed sufficient. From what we know today, a longer period of treatment may be necessary to show effects on muscle biochemistry.
Although the muscle free glutamine concentrations did not directly follow the plasma concentrations, the two compartments were not totally independent. At the start of the study a statistically weak correlation was seen between the free glutamine concentration in plasma and muscle (r=0.32; P<0.05). After the treatment period the changes in the two compartments also showed a statistical correlation (Fig. 3), which may be interpreted as an effect of exogenous glutamine supply even though the period of supplementation was too short.
In order to generate hypotheses for how to design future studies concerning muscle protein depletion in ICU patients, we allowed ourselves to combine the glutamine-treated groups and compare them with the controls. We then found that the 3-methylhistidine excretions in urine decreased by 30% (Fig. 2a), and that the continuing increase in the muscle lactate level seen in the control group was attenuated in the combined glutamine treated groups. The post-hoc finding of a lower 3-methylhistidine excretion in urine suggests that external provision of glutamine decreased the rate of muscle protein degradation, which should be addressed in a study designed for this particular question. There are experimental data in favor of such a possibility [25]. The possibility that a longer period of glutamine treatment is needed cannot be totally ruled out by the present protocol.
It is known that glutathione concentration in muscle of ICU patients is decreased and, in addition, there is a larger fraction of oxidized glutathione compared with healthy individuals [26]. When the muscle glutathione concentration of the patients in this study, before treatment with glutamine, were put in relation to length of ICU stay, an increase was seen during the initial week of ICU stay (Fig. 4). In parallel, a reduction over time in the oxidized fraction of glutathione was seen during the same period (r=0.50; P<0.002; n=34). Furthermore the change in muscle glutamine concentrations during the five-day study period correlated with the change in muscle protein content. The change in muscle glutamate concentration correlated statistically with the change in muscle total glutathione as well as with the red-ox status of glutathione (r=0.40; P<0.02; n=38). These descriptive findings indicate that glutamine depletion in muscle is related to the reduction of muscle glutathione observed in ICU patients, and that if we can find means to attenuate muscle glutamine depletion we may have a possibility to attenuate muscle protein depletion. The increase in muscle glutathione during the initial period of ICU stay is in accord with findings reported elsewhere [27], and also to the relationship between the change in glutamate concentration and the change in total glutathione concentration (r=0.35; P<0.05; n=34) [26].
In summary, glutamine supplementation in three different doses normalized plasma glutamine levels in a dose-dependent way in ICU patients with multiple organ failure. This may explain the beneficial results on patient outcome reported following glutamine supplementation, as glutamine availability increases. Other tissues than skeletal muscle may then make use of the provided glutamine. However, even if given in large doses, glutamine did not attenuate the depletion of muscle free glutamine seen in ICU patients. The observations made in this study raise a number of questions that need to be answered in future studies. Can a restoration of plasma glutamine concentration back to normal by exogenous supplementation during a longer period of time save muscle proteins in ICU patients? Is there a direct relation between glutamine and muscle protein breakdown?
References
Novak F, Heyland DK, Avenell A, Drover JW, Su X (2002) Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med 30:2022–2029
Griffiths RD, Jones C, Palmer TE (1997) Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition 13:295–302
Wischmeyer PE, Lynch J, Liedel J, Wolfson R, Riehm J, Gottlieb L, Kahana M (2001) Glutamine administration reduces Gram-negative bacteremia in severely burned patients: a prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med 29:2075–2080
Goeters C, Wenn A, Mertes N, Wempe C, Van Aken H, Stehle P, Bone HG (2002) Parenteral L-alanyl-L-glutamine improves 6-month outcome in critically ill patients. Crit Care Med 30:2032–2037
Houdijk AP, Rijnsburger ER, Jansen J, Wesdorp RI, Weiss JK, McCamish MA, Teerlink T, Meuwissen SG, Haarman HJ, Thijs LG, van Leeuwen PA (1998) Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet 352:772–776
Mertes N, Schulzki C, Goeters C, Winde G, Benzing S, Kuhn KS, Van Aken H, Stehle P, Furst P (2000) Cost containment throughL-alanyl-L-glutamine supplemented total parenteral nutrition after major abdominal surgery: a prospective randomized double-blind controlled study. Clin Nutr 19:395–401
Jiang ZM, Wang LJ, Qi Y, Liu TH, Qiu MR, Yang NF, Wilmore DW (1993) Comparison of parenteral nutrition supplemented withL-glutamine or glutamine dipeptides. JPEN J Parenter Enteral Nutr 17:134–141
Roth E (1982) Metabolic disorder in severe abdominal sepsis: glutamine deficiency in skeletal muscle. Clin Nutr 1:25–41
Gamrin L, Essen P, Forsberg AM, Hultman E, Wernerman J (1996) A descriptive study of skeletal muscle metabolism in critically ill patients: free amino acids, energy-rich phosphates, protein, nucleic acids, fat, water, and electrolytes. Crit Care Med 24:575–583
Mittendorfer B, Gore DC, Herndon DN, Wolfe RR (1999) Accelerated glutamine synthesis in critically ill patients cannot maintain normal intramuscular free glutamine concentration. JPEN J Parenter Enteral Nutr 23:243–250
Roth E, Karner J, Ollenschlager G (1990) Glutamine: an anabolic effector? JPEN J Parenter Enteral Nutr 14:130S-136S
Gamrin L, Essen P, Hultman E, Wernerman J (1993) Muscle energy status in critically ill patients supplemented with glutamine/alpha-ketoglutarate. Clin Nutr 12 (Suppl 2):17
Karner J, Roth E, Ollenschlager G, Furst P, Simmel A (1989) Glutamine-containing dipeptides as infusion substrates in the septic state. Surgery 106:893–900
Tjader I, Essen P, Thorne A, Garlick PJ, Wernerman J, McNurlan MA (1996) Muscle protein synthesis rate decreases 24 hours after abdominal surgery irrespective of total parenteral nutrition. JPEN J Parenter Enteral Nutr 20:135–138
McNurlan MA, Essen P, Heys SD, Buchan V, Garlick PJ, Wernerman J (1991) Measurement of protein synthesis in human skeletal muscle: further investigation of the flooding technique. Clin Sci (Lond) 81:557–564
Kedenburg CP (1971) A lithium buffer system for accelerated single-column amino acid analysis in physiological fluids. Anal Biochem 40:35–42
Luo JL, Hammarqvist F, Cotgreave IA, Lind C, Andersson K, Wernerman J (1995) Determination of intracellular glutathione in human skeletal muscle by reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Appl 670:29–36
Forsberg AM, Nilsson E, Werneman J, Bergstrom J, Hultman E (1991) Muscle composition in relation to age and sex. Clin Sci (Lond) 81:249–256
Wassner SJ, Schlitzer JL, Li JB (1980) A rapid, sensitive method for the determination of 3-methylhistidine levels in urine and plasma using high-pressure liquid chromatography. Anal Biochem 104:284–289
Millward DJ, Garlick PJ, James WP, Nnanyelugo DO, Ryatt JS (1973) Relationship between protein synthesis and RNA content in skeletal muscle. Nature 241:204–205
Oudemans-van Straaten HM, Bosman RJ, Treskes M, van der Spoel HJ, Zandstra DF (2001) Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med 27:84–90
Essen P, McNurlan MA, Gamrin L, Hunter K, Calder G, Garlick PJ, Wernerman J (1998) Tissue protein synthesis rates in critically ill patients. Crit Care Med 26:92–100
Gamrin L, Essen P, Hultman E, McNurlan MA, Garlick PJ, Wernerman J (2000) Protein-sparing effect in skeletal muscle of growth hormone treatment in critically ill patients. Ann Surg 231:577–586
Essen P, McNurlan MA, Wernerman J, Vinnars E, Garlick PJ (1992) Uncomplicated surgery, but not general anesthesia, decreases muscle protein synthesis. Am J Physiol 262:E253–260
MacLennan PA, Smith K, Weryk B, Watt PW, Rennie MJ (1988) Inhibition of protein breakdown by glutamine in perfused rat skeletal muscle. FEBS Lett 237:133–136
Hammarqvist F, Luo JL, Cotgreave IA, Andersson K, Wernerman J (1997) Skeletal muscle glutathione is depleted in critically ill patients. Crit Care Med 25:78–84
Fläring UB, Rooyackers OE, Werneman J, Hammarqvist F (2003) Temporal changes in muscle glutathione in ICU patients. Intensiv Care Med 29:2193–2198
Hammarqvist F, Stromberg C, von der Decken A, Vinnars E, Wernerman J (1992) Biosynthetic human growth hormone preserves both muscle protein synthesis and the decrease in muscle-free glutamine, and improves whole-body nitrogen economy after operation. Ann Surg 216:184–191
Garlick PJ, McNurlan MA, Essen P, Wernerman J (1994) Measurement of tissue protein synthesis rates in vivo: a critical analysis of contrasting methods. Am J Physiol 266:E287–297
Gamrin L, Berg HE, Essen P, Tesch PA, Hultman E, Garlick PJ, McNurlan MA, Wernerman J (1998) The effect of unloading on protein synthesis in human skeletal muscle. Acta Physiol Scand 163:369–377
Soderlund K, Hultman E (1986) Effects of delayed freezing on content of phosphagens in human skeletal muscle biopsy samples. J Appl Physiol 61:832–835
Acknowledgements
The study was supported by grant from the Swedish Medical Research Council (project 04210). The authors would also like to acknowledge the excellent nursing skill of Ms. V. Gustavsson, and the excellent technical assistance of Ms. E. Nilsson at the Department of Clinical Chemistry, Huddinge University Hospital, Mr. G. Casella at the Department of Surgery, State University of New York, Stony Brook, N.Y., and Ms. L. Thunblad, Ms. E.Skoog-Nejman, Ms. C. Hebert at the Anesthesiological Metabolism Unit, Clinical Research Center, Novum, Huddinge University Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tjäder, I., Rooyackers, O., Forsberg, AM. et al. Effects on skeletal muscle of intravenous glutamine supplementation to ICU patients. Intensive Care Med 30, 266–275 (2004). https://doi.org/10.1007/s00134-003-2048-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-2048-9