Abstract
Objective
This study examined whether continuous hemofiltration favorably affects cardiopulmonary variables, lung inflammation, and lung fluid balance in a canine model of oleic acid induced acute lung injury.
Methods
Eleven pentobarbital-anesthetized dogs were randomly divided into a control (mechanical ventilation, MV) group (n=6) and a MV plus hemofiltration (HF) group (n=5). All animals received an intravenous injection of oleic acid (0.09 ml/kg) to induce acute lung injury. Continuous arterial-venous hemofiltration (blood flow 100 ml/min, ultrafiltration rate at 50–65 ml kg−1 h−1) was started after establishment of oleic acid induced acute lung injury and continued for 4 h. Hemodynamics, lung mechanics, gas exchange, lung fluid balance, lung histology, and the level of plasma cytokines were assessed.
Results
After 240 min of HF treatment there was a significant increase in cardiac output, reduction in pulmonary arterial pressure, and improvement in both oxygenation and lung mechanics. Also, in the HF group the lung wet-to-dry weight ratio was significantly reduced. Histologically, HF reduced edema and inflammatory cell infiltration in the lung. There was also a significantly greater decrease in plasma IL-6 and IL-8 levels in the HF group than in group receiving MV alone.
Conclusions
In a canine model of acute lung injury continuous HF improved cardiopulmonary function, reduced pulmonary edema, decreased lung permeability and inflammation, and decreased the plasma concentration of proinflammatory cytokines.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome is a complex syndrome of acute lung inflammation characterized by alveolar leukocyte infiltration and protein-rich pulmonary edema that is associated with acute respiratory failure. Numerous inflammatory mediators have been identified, and these mediators may play an important role in the initiation and progression of the acute respiratory distress syndrome [1]. Although numerous therapies have been tested, only a lung protective low tidal volume strategy has been proven to be effective in reducing mortality [2]. Therefore new therapies are needed.
Recently hemofiltration (HF) has been investigated as a potential supportive therapy that might be effective in removing a number of circulating proinflammatory molecules in clinical sepsis [3, 4, 5, 6, 7, 8]. Several studies have evaluated the effects of HF on hemodynamics during septic shock in animal models [9, 10, 11, 12]. Therefore, we hypothesized that HF would reduce the concentrations of inflammatory mediators in the plasma and correct the instability of hemodynamics and reduce lung inflammation in experimental acute lung injury (ALI).
Oleic acid induced ALI in dogs is manifested by pulmonary hypertension, profound hypoxemia, and marked cardiovascular instability. Intravenous injection of oleic acid can trigger neutrophil activation, injury of capillary endothelium, and release of proinflammatory mediators [13, 14, 15], which in its initial stages resembles human acute respiratory distress syndrome histologically and physiologically [13]. To test our hypothesis, we studied the effect of HF on hemodynamics, lung mechanics, gas exchange, lung fluid balance, lung histology, and the level of plasma cytokines in oleic acid induced lung injury. The results of this study provide preclinical evidence for a potential beneficial effect of HF on the course of ALI.
Materials and methods
Animals
Adult mongrel dogs of both sexes weighing 14.3±4.2 kg were purchased from Animal Center of Fudan University (Shanghai, China). Dogs were anesthetized by intravenous injection of pentobarbital sodium (30 mg/kg) followed by infusion 4 mg kg−1 h−1 throughout the experiment. After the trachea was intubated with a number 8 cuffed endotracheal tube, the dogs were stabilized for 30 min before the experiment. The animal care and use committee of Fudan University approved the protocol.
Ventilation and measurements of lung mechanics
The dogs were ventilated by Servo 900C ventilator (Siemens-Elema, Solna, Sweden) with synchronized intermittent mechanical ventilation (MV), with a frequency of 17 breaths/min, tidal volume of 10 ml/kg, and inspiratory time fraction of 1:2. FIO2 was 0.21 at baseline and until ALI was diagnosed. Then FIO2 was increased to 0.4. Expiratory tidal volume, peak airway pressure, and dynamic respiratory compliance were recorded through a lung mechanics monitor connected to the endotracheal tube (Novametrix Medical Systems, Wallingford, Conn., USA).
Hemodynamics and blood gas analysis
Surgery was carried out in a sterile fashion, with dogs in the supine position. Femoral artery and vein catheters were inserted. Catheter patency was maintained with intermittent flushes of heparinized saline. Systemic mean arterial pressure and heart rate were recorded from a femoral artery catheter (Deseret Medical, Sandy, Utah, USA). A pulmonary artery catheter (7-F, heparin-coated thermodilution catheter, Arrow International, Pa., USA) was introduced via a femoral vein for measurement of pulmonary artery pressure and pulmonary artery occlusion pressure. Pressure tracings (Hewlett-Packard Company, Loveland, Colo., USA) were used to verify the correct positions of the proximal and distal ports of the pulmonary artery catheter. Cardiac output was measured by thermodilution technique with 5-ml bolus injections of 0.9% saline at room temperature delivered during expiration. A difference between two successive measurements less than 10% was obtained to calculate mean values. All intravascular pressures were measured with low-displacement transducers (Arrow International) referenced to the midchest level. Arterial and mixed venous blood gases were analyzed at 37°C with a blood gas analyzer (Diamond Diagnostics, Holliston, Mass, USA) and were corrected to core temperature as measured with the thermistor at the distal end of the pulmonary artery catheter. Stroke volume was calculated. Left ventricular stroke work was calculated as the product of stroke volume and the difference between mean arterial pressure and pulmonary artery occlusion pressure. Venous admixture was calculated with standard formulas.
Hemofiltration setting
The HF device consisted of a roller pump, air detector, bubble trap, and pressure feedback system (Diapact CCRT, Braun, Melsungen, Germany). A 0.4 m2 polysulfone hollow-fiber with a molecular weight cutoff of 40,000 kDa (AV-400, Fresenius, Germany) was used. The filter was circulated with normal saline containing heparin (5,000 IU/l) for 30 min before connected to the circuit. Zero-balanced HF was achieved with blood flow at 100 ml/min. Blood was drained from the right femoral artery and returned through the left femoral vein. The replacement of buffer solution contained 30 mmol/l bicarbonate, 2.9 mmol/l lactate, and other electrolytes (Changzheng Pharmaceutical, Shanghai, China) and was warmed to 37°C and infused before the filter at a rate of 50–65 ml kg−1 h−1. The balance computer automatically compensated for variance in fluid filtration and substitution. During the 240 min of HF, the pre-filter administration of heparin (1,500 IU/h) was used to anticoagulate the circuit.
In the preliminary control experiments to investigate whether the extracorporeal circuit would affect the hemodynamics and lung mechanics the polysulfone hemofilter was replaced by a piece of plastic tubing of the same length. The circuit was primed, and blood was pumped and anticoagulated in the same fashion as for HF, but no ultrafiltrate was generated and no replacement fluid was administered. Four oleic acid challenged dogs were connected with extracorporeal circuit and ventilated under the same conditions as the MV group. There were no significant differences in hemodynamics and lung mechanics between MV plus sham and the MV group.
Model of acute lung injury
After recording hemodynamics, gas exchange, and lung mechanics at baselineALI was induced by injecting oleic acid (Yuanhang Reagent, Shanghai, China; 0.09 ml/kg) into the right atrium via the proximal port of the pulmonary arterial catheter. The route of injection and the dose of oleic acid were chosen on the basis of preliminary experiments to achieve a level of lung injury similar to that in prior studies [16, 17]. When the PaO2 was less than 60 mmHg at an FIO2 0.21, ALI was established according to the criteria in previous reports [16, 17, 18]. Oleic acid induced ALI developed and stabilized by 120–180 min after administration of oleic acid. When the ALI model was established, dogs were ventilated with room air. After ALI developed, the FIO2 was increased to 0.40. Intermittent blood gas analyses were carried out at intervals of 20–30 min to determine when ALI was established.
Experimental protocols
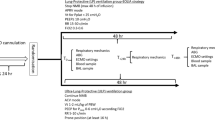
After oleic acid induced lung injury had developed, the dogs were randomly divided into a group receiving only MV (n=6) and a group receiving MV plus HF (n=5). The experimental time line of the study is summarized in Fig. 1.
Baseline designates the beginning of the experiment after anesthesia and stabilization. The dogs spontaneously breathed room air or were ventilated with an FIO2 0.21 to maintain oxygen saturation at normal levels (SpO2 ≥90%). Hemodynamics, lung mechanics, and arterial blood gases were measured. ALI 0 min indicates that oleic acid induced acute lung injury was established. The diagnosis of ALI was based on a PaO2 value less than 60 mmHg with an FIO2 0.21. After recording cardiopulmonary parameters, the dogs were ventilated with an FIO2 0.4 in the control or MV plus zero-balanced hemofiltration in the treated group. At ALI 120 min blood and BAL samples were collected. At 230 min blood and BAL samples were obtained. The dogs were killed at 240 min with pentobarbital, and postmortem lung samples were obtained
Albumin measurement in bronchoalveolar lavage fluid
Bronchoalveolar lavage (BAL) was performed 120 and 230 min after the onset of ALI by advancing a catheter into the distal airways of the right lower lobe. Normal saline (25 ml) warmed to body temperature was instilled and aspirated. The fluid recovery was 80–90% in both groups. The BAL was filtered through 0.4-µm Millipore filter to remove debris and then centrifuged at 2000 rpm. The supernatant was stored at −70°C to measure protein concentration by the Bradford method [19]. The ratio between BAL fluid urea and serum urea was used to calculate the dilution of the original protein concentration by BAL fluid [20].
Evaluation of lung fluid balance
After the experiment protocol was completed all dogs were rapidly exsanguinated over 5 min. The hili were clamped, and the right and left lungs were excised. The exsanguinated total lung wet weight was measured. Approximately 10-g samples were obtained from the left lower lobe and were desiccated in an oven at 55°C for 72 h to determine the lung wet-to-dry weight ratio.
Lung histopathology
Briefly, at least three pieces of lung tissues (approx. 2×1×0.5 cm) obtained from the right and left upper lobe were placed into 10% buffered formalin for 24 h. Lung tissue was embedded in paraffin, and 4-µm sections were cut. Hematoxylin and eosin stained sections were prepared by standard techniques. The degree of microscopic injury was scored based on the following variables: hemorrhage, interstitial edema, necrosis, neutrophil infiltration, and atelectasis. The severity of injury was judged by the following criteria [21]: no injury=0; injury to 25% of the field=1; injury to 50% of the field=2; injury to 75% of the field=3; and diffuse injury=4. A pathologist who was blinded to the experimental protocol provided a score for each variable based on the severity of injury. The sum of all scores was combined to calculate a composite score.
Cytokines measurements
Blood was collected in tubes containing EDTA (1 mg/ml) and centrifuged immediately at 3,000 rpm for 10 min. The plasma was frozen at −70°C for later analysis. Anti-human enzyme-linked immunosorbent assay kits (Biosource, USA) were used for measurements of plasma interleukins (IL)6 and 8 and of tumor necrosis factor (TNF) α under the same experimental conditions. In preliminary experiments we validated the assays by using diluted human standards and pooled canine plasma over a range of concentrations of cytokines. The results demonstrated there was cross-reactivity between human and canine TNF-α, IL-6, and IL-8.
Statistical analysis
Statistics analyses were performed SPSS software (Chicago, Ill., USA), and the results are presented as mean ±SD. Intragroup comparisons to parameters at baseline and those measured after onset of ALI were assessed by the t test for paired samples and one-way analysis of variance. The unpaired t test was used to test the difference in protein concentrations of BAL and the lung wet-to-dry weight ratios. The Mann-Whitney test was used to access the significance of the histological scores.
Results
Hemodynamics
There was a marked increase in pulmonary arterial pressure at the onset of ALI in both groups (p<0.01; Fig. 2A). There was significantly lower pulmonary arterial pressure at 120 (28±3 mmHg) and 240 min (26±4 mmHg) in the MV plus HF group than in the MV alone group at 120 (42±3 mmHg) and 240 min (37±2 mmHg; p<0.01). The pulmonary artery occlusion pressure showed no significant changes during the course of ALI (Fig. 2B).
Effect of hemofiltration on hemodynamics. A pulmonary artery pressure. *p<0.01 vs. baseline in both groups, † p<0.01 at 120 min in MV plus HF group vs. 120 min in MV alone group, # p<0.01 at 240 min in MV plus HF group vs. 0 min and 240 min in MV alone group. B No change was found in pulmonary artery occlusion pressure. C HF improved CO, *p<0.05 vs. baseline, † p<0.05 at 240 min in MV plus HF group vs. 240 min in MV alone group, # p<0.05 240 min vs. 0 min in MV alone group. D Left ventricular stroke work. *p<0.05 at 240 min in the HF plus MV group vs. 240 min in the MV alone group. E, F No change was found in mean arterial pressure and heart rate. Values are means ±SD; n=6 in the MV alone group; n=5 in the MV plus HF group
Cardiac output in both the MV and MV plus HF groups significantly declined at the onset of ALI (p<0.01; Fig. 2C). A further decrease in cardiac output (0.87±0.15 l/min) occurred at 240 min in the MV alone group. In contrast, at 240 min cardiac output (1.45±0.35 l/min) was significantly higher in the MV plus HF group. Left ventricular stroke work (20±7 g m−1 min−1) was significantly higher in the MV plus HF group than the MV alone group (10±4 g m−1 min−1) at 240 min (p<0.01; Fig. 2D). HF had no impact on systemic arterial pressure or heart rate (Fig. 2E, F).
Lung mechanics
Peak respiratory airway pressure was significantly lower at 240 min (9±2 cmH2O) in the MV plus HF group (p<0.05; Fig. 3A). Also, lung compliance in the MV plus HF group (1.28±0.28 ml cmH2O−1 kg−1) was significantly higher at 240 min than in MV alone group (0.77±0.42 ml cmH2O−1 kg−1; p<0.05; Fig. 3B).
Effect of hemofiltration on lung mechanics and oxygenation. A Peak inspiratory airway pressure. B Respiratory compliance. *p<0.05 ALI at 240 min vs. ALI at 0 min in the MV plus HF group, # p<0.05 ALI at 240 min in the MV plus HF group vs. at 240 min in the MV alone group. C PaO2 and D Venous admixture. *p<0.05 MV plus HF at 240 min vs. MV at 240 min, **p<0.01 ALI at 0 min vs. baseline in both groups, # p<0.01 0 min vs. 240 min in the MV plus HF group. FIO2 was 0.21 for baseline and ALI at 0 min. Values are means ±SD; n=6 in the MV alone group; n=5 in the MV plus HF group
Oxygenation and venous admixture
PaO2 decreased below baseline at the onset of ALI to an equivalent level in the two groups (p<0.01; Fig. 3C). MV did not significantly elevate the PaO2 at either 120 min or 240 min (p>0.05). However, PaO2 was significantly higher in the MV plus HF group (107±6 mmHg) at 240 min than in the MV alone group (70±8 mmHg; p<0.01). The venous admixture was also significantly better at 240 min with MV plus HF (9±2%) than with MV alone (15±4%; p<0.01; Fig. 3D).
Soluble mediators in plasma
Plasma IL-6 was significantly lower at 240 min in the MV plus HF group (18±7 pg/ml) than in the MV alone group (35±8 pg/ml; p<0.05; Fig. 4A). There was also a lower plasma IL-8 level at 240 min (4±3 pg/ml) with MV plus HF than with MV alone (16±8 pg/ml; p<0.01; Fig. 4B). There were no changes in plasma TNF-α level in either group (Fig. 4C).
Changes in cytokine levels in the plasma. A IL-6. B IL-8. C TNF-α. *p<0.05 ALI at 0 min vs. at 240 min in the MV alone group, # p<0.05 at 240 min in the MV plus HF group vs. at 240 min in the MV alone group, ## p<0.01 at 240 min in the MV plus HF group vs. at 240 min in the MV alone group. Values are means ±SD; n=6 in the MV group, n=5 in MV plus HF group
Protein concentration of bronchoalveolar lavage fluid
HF reduced permeability to protein in the lung, as reflected by a lower albumin concentration in BAL of the MV plus HF group (Fig. 5A).
A. Albumin concentration of BAL in the MV alone and MV plus HF groups. *p<0.05 MV at 240 min vs. MV plus HF at 240 min. B Effect of hemofiltration on lung fluid balance as measured be the lung wet-to-dry weight ratio in oleic acid induced acute lung injury. *p<0.05 vs. 240 min in MV group. Values are means ±SD; n=6 in the MV group; n=5 in the MV plus HF group
Evaluation of lung water
The wet weight of lungs in the MV plus HF group (327±31 g) was significantly lower than that in the MV alone group (384±21 g; p<0.05). The lung wet-to-dry weight ratio was also significantly lower in the MV plus HF group than the MV alone group (Fig. 5B).
Lung histopathology
HF significantly reduced the lung interstitial edema, neutrophil infiltration, atelectasis, and total lung injury histology score (Fig. 6A). This difference is illustrated in a representative histological section (Fig. 6B, 1–4). Light microscopic findings in the MV alone group included hemorrhage and edema, thickened alveolar septum, formation of hyaline membranes, and the existence of inflammatory cells in alveolar spaces (Fig. 6B, 1–2). In HF group these changes were far less marked than in the MV alone group (Fig. 6B, 3–4).
Histological changes in oleic acid injured lungs treated with MV alone or MV plus HF; hematoxylin-eosin stain. A Lung injury score in the two groups. *p<0.05 at 240 min in the MV plus HF group vs. 240 min in the MV alone group. B Representative histological changes after 4 h in the MV alone or MV plus HF group. 1, 2 MV alone group; 3, 4 MV plus HF group; bar 50 µm, magnification ×100; 200 µm, magnification ×400)
Discussion
The potential efficacy of HF for treating ALI is based in part on the hypothesis that HF will remove humoral mediators of ALI, resulting in less lung edema. To test this hypothesis we used a well established animal model of oleic acid induced ALI to study the effects of HF on systemic and pulmonary hemodynamics, gas exchange, lung mechanics, lung fluid balance, lung histology, and the clearance of soluble mediators in the bloodstream.
Oleic acid infusion generated ALI with sustained arterial hypoxemia, pulmonary hypertension, compromised cardiovascular performance [22], and lung edema [16]. Therefore we used this canine model to test the hypothesis that HF would improve cardiopulmonary parameters, reduce lung edema, and favorably alter the balance of pro- and anti-inflammatory cytokines. The present experimental study showed that HF improves cardiac output, stroke volume, and left ventricular stroke work. These results show that HF is beneficial for hemodynamics in oleic acid induced ALI. The beneficial effects of HF on pulmonary and cardiac function have been reported in several previous studies in animal [11, 12, 23] and human studies [24, 25]. Boga et al. [26] found that cardiac output, cardiac index, and systemic vascular resistance values are significantly improved in hemofiltered patients with sepsis. Grootendorst et al. [27] concluded that high volume HF improves right ventricular function by removal of vasoactive mediators that are responsible in part for myocardial depression in endotoxin-induced shock in the pig. Hemofiltration improved hemodynamics by improving contractility and possibly by reducing myocardial edema in children following cardiac surgery [28].
The data from this study demonstrate that HF reduces the severity of experimental ALI by several physiological gravimetric and histological criteria (Fig. 6A, B). There are two possible mechanisms for the beneficial effects: HF reduces lung vascular hydrostatic forces and/or lung vascular permeability. Pulmonary arterial pressure was significantly decreased by 9–10 mmHg in the MV plus HF group after 4 h of treatment, although there was no change in pulmonary artery occlusion pressure (a surrogate for left atrial pressure). This reduction in pulmonary artery pressure may have reduced lung vascular pressure, providing one possible mechanism for the reduction in lung water with the HF treatment. There was also a decline in lung permeability, reflected by a lower albumin concentration in the BAL of the MV plus HF group. Lung histology also demonstrated that there were fewer inflammatory cells and less alveolar injury in the MV plus HF group than in the MV group. The most likely explanation for the beneficial effect on lung permeability was that HF removed proinflammatory cytokines from the plasma.
IL-6 and IL-8 seem to play a role in clinical and experimental ALI [14, 29, 30]. IL-8 binds to specific receptors on neutrophils and augments their migration and degranulation to participate in ALI. Elimination of IL-8 in the plasma would decrease the migratory signal for and activation of polymorphonuclear neutrophils. Generally the molecular weights of both IL-6 (21 kDa) and IL-8 (8 kDa) are under the cutoff point of the hemofilter; the removal of plasma IL-6 and IL-8 may be through either convection or adsorption, or both. Some data suggest that adsorption is the most important clearance mechanism, especially immediately after the start of HF [31, 32]. The rapid early decline in plasma concentration strongly suggests membrane adsorption as the major mechanism for mediator removal [33]. In the present study HF plus MV significantly decreased the plasma levels of IL-6 and IL-8 at 240 min compared to MV alone. This may be a mechanism to explain the reduction in lung injury in the MV plus HF group. In a pig model with endotoxin-induced ALI HF improved arterial oxygenation and lung function independently of the improvement in hemodynamics, fluid removal, and body temperature [34]. Some clinical studies have reported that HF reduced IL-6 and IL-8 concentrations in the plasma, findings that were associated with improved outcomes in the patients with sepsis and multiple organ failure [24, 33, 35, 36, 37, 38]. Experimental studies also demonstrated that removal of cytokines by HF was related to a better prognosis [9, 32].
TNF-α was not significantly changed in either group, suggesting that TNF-α is not altered during the short-term of oleic acid induced ALI. Others have found that TNF-α levels do not change in a piglet model of oleic acid induced ALI [22].
In the present study the HF was performed in the early phase of ALI. Recent findings have shown that removal of proinflammatory cytokines by prophylactic HF or HF in the early phase of disease may be associated with better clinical outcomes [9, 24, 35, 37, 39]. Therefore early elimination of cytokines in the oleic acid induced ALI dogs may have contributed to reduced lung vascular permeability and lung water and to the improved cardiac function.
In summary, continuous HF improved cardiopulmonary function and reduced pulmonary edema and the severity of oleic acid induced lung injury in dogs, probably because of both a reduction in hydrostatic pressure (lower pulmonary arterial pressure) and a reduction in lung vascular permeability, perhaps in part by removing proinflammatory cytokines from the plasma.
References
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL (2003) Future research directions in acute lung injury: summary of a national heart, lung, and blood institute working group. Am J Respir Crit Care Med 167:1027–1035
Bauer M, Marzi I, Ziegenfuss T, Riegel W (2001) Prophylactic hemofiltration in severely traumatized patients: effects on post-traumatic organ dysfunction syndrome. Intensive Care Med 27:376–383
Bellomo R, Tipping P, Boyce N (1995) Interleukin-6 and Interleukin-8 extraction during continuous venovenous hemofiltration in septic acute renal failure. Ren Fail 17:457–466
Boga M, Islamoglu Badak I, Cikirikcioglu M, Bakalim T, Yagdi T, Buket S, Hamulu A (2000) The effects of modified hemofiltration on inflammatory mediators and cardiac performance in coronary artery bypass grafting. Perfusion 15:143–150
Cole L, Bellomo R, Journois D, Davenport P, Baldwin I, Tipping P (2001) High-volume haemofiltration in human septic shock. Intensive Care Med 27:978–986
De Vriese AS, Colardyn FA, Philippe JJ, Vanholder RC, De Sutter JH, Lameire NH (1999) Cytokine removal during continuous hemofiltration in septic patients. J Am Soc Nephrol 10:846–853
De Vriese AS, Vanholder RC, Pascual M, Lameire NH, Colardyn. FA (1999) Can inflammatory cytokines be removed efficiently by continuous renal replacement therapies? Intensive Care Med 25:903–910
Mink SN, Li X, Bose D, Gu M, Liu G, Jacobs H, Light RB (1999) Early but not delayed continuous arteriovenous hemofiltration improves cardiovascular function in sepsis in dogs. Intensive Care Med 25:733–743
Rogiers P, Zhang H, Smail N, Pauwels D, Vincent JL (1999) Continuous venovenous hemofiltration improves cardiac performance by mechanisms other than tumor necrosis-α attenuations during endotoxic shock. Crit Care Med 27:1848–1855
Stein B, Pfenniger E, Grunert A, Schmitz JE, Hudde M (1990) Influence of continuous hemofiltration on hemodynamics and central blood volume in experimental endotoxic shock. Intensive Care Med 16:494–499
Stein B, Pfenniger E, Grunert A, Schmitz AJE, Deller A, Kocher F (1991) The consequences of continuous hemofiltration on lung mechanics and extravascular lung water in a porcine endotoxic shock model. Intensive Care Med 17:293–298
Syrbu S, Thrall RS, Smilowitz HM (1996) Sequential appearance of inflammatory mediators in rat bronchoalveolar lavage fluid after oleic acid-induced lung injury. Exp Lung Res 22:33–49
Furue S, Kuwabara K, Mikawa K, Nishina K, Shiga M, Maekawa N, Ueno M, Chikazawa Y, Ono T, Hori Y, Matsukawa A, Yoshinaga M, Obara H (1999) Crucial role of group IIA phospholipase A (2) in oleic acid-induced acute lung injury in rabbits. Am J Respir Crit Care Med 160:1292–1302
Kuwabara K, Furue S, Tomita Y, Ueno M, Ono T, Matsukawa A, Yoshinaga M, Mikawa K, Nishina K, Shiga M, Obara H, Hori Y (2001) Effect of methylprednisolone on phospholipase A (2) activity and lung surfactant degradation in acute lung injury in rabbits. Eur J Pharmacol 433:209–216
Mitaka C, Hirata Y, Habuka K, Narumi Y, Yokoyama K, Makita K, Imai T (2002) Atrial natriuretic peptide improves pulmonary gas exchange by reducing extravascular lung water in canine model with oleic acid-induced pulmonary edema. Crit Care Med 30:1570–1575
Neumann P, Berglund JE, Andersson LG, Maripu E, Magnusson A, Hedenstierna G (2000) Effects of inverse ratio ventilation and positive end-expiratory pressure in oleic acid-induced lung injury. Am J Respir Crit Care Med 161:1537–1545
Abraham E, Matthay MA, Dinarello CA, Vincent JL, Cohen J, Opal SM, Glauser M, Parsons P, Fisher CJ Jr, Repine JE (2000) Consensus conference definitions for sepsis, septic shock, acute lung injury, and acute respiratory distress syndrome: time for a reevaluation. Crit Care Med 28:232–235
Zor T, Selinger Z (1996) Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem 236:302–308
Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, Crystal RG (1986) Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60:532–538
Jeng MJ, Kou YR, Sheu CC, Hwang B (2002) Effects of partial liquid ventilation with FC-77 on acute lung injury in newborn piglets. Pediatr Pulmonol 33:12–21
Rosenthal C, Caronia C, Quinn C, Lugo N, Sagy M (1998) A comparison among animal models of acute lung injury. Crit Care Med 26:912–916
Gomez A, Wang R, Unruh H, Light RB, Bose D, Chau T, Correa E, Mink S (1990) hemofiltration reverses left ventricular dysfunction during sepsis in dogs. Anesthesiology 73:671–685
Honore PM, Jamez J, Wauthier M, Lee PA, Dugernier T, Pirenne B, Hanique G, Matson JR (2000) Prospective evaluation of short-term, high-volume isovolemic hemofiltration on the hemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit Care Med 28:3581–3587
Reeves JH, Butt WW, Shann F (1999) Continuous plasmafiltration in sepsis syndrome. Plasmafiltration in Sepsis Study Group. Crit Care Med 27:2096–2104
Boga M, Islamoglu Badak I, Cikirikcioglu M, Bakalim T, Yagdi T, Buket S, Hamulu A (2000) The effects of modified hemofiltration on inflammatory mediators and cardiac performance in coronary artery bypass grafting. Perfusion 15:143–150
Grotendorst AF, van Bommel EFH, Van der Hoven B, van Osta ALM (1992) High volume hemofiltration improves right ventricular function in endotoxin-induced shock in the pig. Intensive Care Med 18:235–240
Rivera ES, Kimball TR, Bailey WW, Witt SA, Khoury PR, Daniels SR (1998) Effect of veno-venous ultrafiltration on myocardial performance immediately after cardiac surgery in children. A prospective randomized study. J Am Coll Cardiol 32:766–772
Miller EJ, Cohen AB, Nagao S, Griffith D, Maunder RJ, Martin TR, Weiner-Kronish JP, Sticherling M, Christophers E, Matthay MA (1992) Elevated levels of NAP-1/interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am Rev Respir Dis 146:427–432
Tyburski JG, Dente C, Wilson RF, Steffes C, Devlin J, Carlin AM, Flynn LM, Shanti C (2001) Differences in arterial and mixed venous IL-6 levels: the lungs as a source of cytokine storm in sepsis. Surgery 130:748–751
De Vriese AS, Vanholder RC, Pascual M, Lameire NH, Colardyn. FA (1999) Can inflammatory cytokines be removed efficiently by continuous renal replacement therapies? Intensive Care Med 25:903–910
Kellum JA, Dishart MK (2002) Effect of hemofiltration filter adsorption on circulating IL-6 levels in septic rats. Crit Care 6:429–433
Cole L, Bellomo R, Journois D, Davenport P, Baldwin I, Tipping P (2001) High-volume haemofiltration in human septic shock. Intensive Care Med 27:978–986
Ullrich R, Roeder G, Lorber C, Quezado ZM, Kneifel W, Gasser H, Schlag G, Redl, Germann P (2001) Continuous venovenous hemofiltration improves arterial oxygenation in endotoxin-induced lung injury in pigs. Anesthesiology 195:428–436
Bauer M, Marzi I, Ziegenfuss T, Riegel W (2001) Prophylactic hemofiltration in severely traumatized patients: effects on post-traumatic organ dysfunction syndrome. Intensive Care Med 27:376–383
Bellomo R, Tipping P, Boyce N (1995) Interleukin-6 and Interleukin-8 extraction during continuous venovenous hemofiltration in septic acute renal failure. Ren Fail 17:457–466
Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamura M (2002) Continuous hemofiltration/hemodiafiltration in critical care. Ther Apher 6:193–198
Wakabayashi Y, Kamijou Y, Soma K, Ohwada T (1996) Removal of circulating cytokines by continuous hemofiltration in patients with systemic inflammatory response or multiple organ dysfunction syndrome. Br J Surg 83:393–394
Journois D, Israel-Biet D, Pouard P, Rolland B, Silvester W, Vouhe P, Safran D (1996) High-volume, zero-balanced hemofiltration to reduce delayed inflammatory response to cardiopulmonary bypass in children. Anesthesiology 85:965–976
Acknowledgements
This study was supported by a grant from the Committee of Science and Technology of Shanghai (O3D219619) and in part by National Institutes of Health Grant HL 51854. The authors thank Miss J. Li for technical assistance and supply of reagents, Mr. Qin for care of the animals, Dr. Z.Y. Ren and S. Zhang PhD for helpful discussions, and Senxiong Immunol Lab for the enzyme-linked immunosorbent assay kits.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, X., Bai, C., Hong, Q. et al. Effect of continuous hemofiltration on hemodynamics, lung inflammation and pulmonary edema in a canine model of acute lung injury. Intensive Care Med 29, 2034–2042 (2003). https://doi.org/10.1007/s00134-003-2017-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-2017-3