Abstract
Objective
To assess the acute effect of a lung recruitment maneuver (LRM) on lung morphology in patients with acute lung injury (ALI) or acute respiratory distress syndrome (ARDS).
Patients
Ten patients with ALI/ARDS on mechanical ventilation.
Design
Prospective clinical study.
Setting
Computed tomography (CT) scan facility in a teaching hospital.
Interventions
An LRM performed by stepwise increases in positive end-expiratory pressure (PEEP) of up to 30–40 cmH2O. Lung basal CT sections were taken at end-expiration (patients 1 to 5), and at end-expiration and end-inspiration (patients 6 to10). Arterial blood gases and static compliance (Cst) were measured before, during and after the LRM.
Measurements and main results
Poorly aerated and non-aerated tissue at PEEP 10 cmH2O accounted for 60.0±29.1% of lung parenchyma, while only 1.1±1.8% was hyperinflated. Increasing PEEP to 20 and 30 cmH2O, compared to PEEP 10 cmH2O, decreased poorly aerated and non-aerated tissue by 16.2±28.0% and 33.4±13.8%, respectively (p<0.05). This was associated with an increase in PaO2 and a decrease in total static compliance. Inspiration increased alveolar recruitment at all PEEP levels. Hyperinflated tissue increased up to 2.9±4.0% with PEEP 30 cmH2O, and to a lesser degree with inspiration. No barotrauma or severe hypotension occurred.
Conclusions
Lung recruitment maneuvers improve oxygenation by expanding collapsed alveoli without inducing too much hyperinflation in ALI/ARDS patients. An LRM during the CT scan gives morphologic and functional information that could be useful in setting ventilatory parameters.
Similar content being viewed by others
Introduction
Computed tomography (CT) has been widely used during the last 20 years to assess lung morphology; this has resulted in a better understanding of the pathophysiology of acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS), and ventilator-induced lung injury [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. At present, there is strong evidence that mechanical ventilation affects the outcome of patients with ALI/ARDS [13, 14, 15, 16]. Optimization of alveolar recruitment and prevention of lung overdistention seem to play a major role in avoiding the stress and damage of mechanical ventilation. Lung recruitment maneuvers (LRMs) are being increasingly used to optimize alveolar recruitment in patients with ALI/ARDS [13, 14]. High but brief peak inspiratory or end-expiratory pressures improve oxygenation without detrimental effects on hemodynamics or barotrauma [17, 18, 19, 20, 21, 22]. However, there are no data on the morphologic changes during or after the LRM, which could shed light on the mechanism involved.
The aim of our study was to assess the acute effect of an LRM on lung morphology, made by stepwise increases in positive end-expiratory pressure (PEEP) during the lung CT scan in patients with ALI/ARDS.
Methods
All patients admitted to our eight-bed surgical ICU who required mechanical ventilation were managed following our ventilatory management protocol. Briefly, this involves 8 ml/kg tidal volume (Vt), PEEP levels 5–8 cmH2O and FIO2 to maintain oxygen saturation (SatO2) above 90% under volume-controlled ventilation as starting parameters. Plateau pressures above 25 cmH2O, oxygenation index greater than 10 cmH2O/mmHg [23] or patients fulfilling ARDS criteria [24] identify patients with severe acute respiratory failure. Pressure-volume (P-V) curves according to the Levy method [25] are traced and PEEP is set 2 cmH2O above the inflection point (Pflex) in these patients. Vt is decreased to 5 ml/kg in order to maintain plateau pressures below 25–30 cmH2O. Prone positioning, differential ventilation, nitric oxide and extracorporeal assistance are used as adjunctive therapies in the more severe patients. Invasive hemodynamic monitoring is performed to attempt fluid restriction and decrease lung water without jeopardizing systemic perfusion. Midazolam and morphine are used as sedatives at the lowest dose that allows gentle ventilation. The weaning period is started on pressure support plus intermittent volume-controlled ventilation. Extubation is accomplished when the patient can breathe normally under low levels of pressure support and PEEP.
Computed tomography scan
Patients requiring lung CT scan for diagnostic or therapeutic purposes were included in the study. Lung CT scan is not routine in ALI/ARDS patients. We have set up the CT scan facility room as an ICU room so we can work on the ventilator and make inspiratory or expiratory pauses with known levels of airway pressure. This avoids patient movement, improves the quality of CT images and allows a better interpretation of them. Patients are connected to a Siemens 900-C (Siemens, Munich, Germany) ventilator with PEEP 10–15 cmH2O, under sedatives and muscle relaxants. Non-invasive hemodynamic monitoring and SatO2 are continuously measured. Informed written consent is obtained from the patient's next of kin to allow the dynamic slices.
At first, a conventional CT scan (Picker PQ2000; Philips Medical Systems, Best, The Netherlands) from the neck down to the lung bases is performed with 8 mm thick CT slices during an inspiratory pause. The matrix is 512×512, which gives a voxel size around 2–3 mm3. After seeing the images on the screen, one CT slice is selected for the dynamic protocol, usually at the middle or lower third of the thorax, which will be later referred to as the basal CT section [4].
Protocol 1
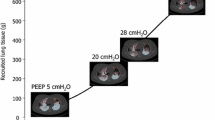
The first five patients were on volume-controlled ventilation for the whole LRM protocol with baseline PEEP from 10 to 14 cmH2O (Fig. 1, Table 1). After the basal CT section was selected, PEEP was switched down to 0 cmH2O (ZEEP) and FIO2 increased to maintain SatO2 above 0.9. The ZEEP basal CT section was taken after 3–5 min during an expiratory pause. PEEP was then increased in 5 cmH2O steps up to 30–40 cmH2O. End-expiratory basal CT sections were taken after 30–45 s at each PEEP level (Fig. 1 and Fig. 3). At high levels of PEEP, and as peak airway pressures were approaching 50 cmH2O, Vt was decreased and respiratory rate increased to maintain minute ventilation. After 4–6 min for the whole LRM, of which 2 or 3 were PEEP levels at or above 20 cmH2O, ventilatory parameters were set to the previous levels.
Pressure-time curve for the first protocol (patients 1 to 5). A lung CT scan is performed at end-inspiration under volume-controlled ventilation. After 3–5 min of zero end-expiratory pressure (ZEEP), a lung recruitment maneuver consisting in 5 cmH2O increments in positive end-expiratory pressure (PEEP) up to 40 cmH2O is performed. The selected basal CT sections were taken at each PEEP level at end-expiration
Protocol 2
For the next five patients, the ventilatory mode was switched to pressure-controlled ventilation, with 20 cmH2O driving pressures and PEEP 10 cmH2O as baseline parameters, and basal CT sections were taken both during an expiratory, and again at an inspiratory pause (Fig. 2). Then PEEP was increased by 10 cmH2O up to 30 cmH20 and basal CT slices were taken at expiratory and inspiratory pauses. Finally, ventilatory parameters were set down to baseline and, after 3–5 min, a new CT slice was taken. Mean airway pressures (MAP) and expiratory Vt were measured on the ventilator and arterial blood gases taken at baseline, at 30 cmH2O PEEP and after the LRM. Static compliance (Cst) was calculated as Cst = Vt /20, and oxygenation index (Ox.Ix.) as Ox.Ix. = (MAP x FIO2) / PaO2 [3]. This new protocol took 2–3 min for the LRM and PEEP was not dropped below 10 cmH2O at any time to avoid loss of recruitment.
Pressure-time curve for the second protocol (patients 6 to 10). A lung CT scan is performed at end-inspiration under pressure-controlled ventilation with 20 cmH2O driving pressure and 10 cmH2O positive end-expiratory pressure (PEEP). Then, a lung recruitment maneuver (LRM) consisting of 10 cmH2O increments in PEEP up to 30 cmH2O is performed. The selected basal CT sections were then taken at each PEEP level and after the LRM at baseline parameters at end-expiration and end-inspiration
Image processing
Computed tomography images are downloaded to an optical disc and processed using Osiris software (University Hospitals of Geneva). We used methods similar to other CT studies [3, 4, 5, 6, 7, 8, 9, 10, 11, 12]. First, the lung contour on each basal CT section was traced and total CT volume (TVCT) and average density (HCT) were measured. With these data, we obtained the amount of gas volume (GVCT = TVCT x HCT / −1000) and weight (WCT = TVCT −GVCT) for each lung slice. Lung parenchyma characteristics were defined according to their densities measured in Hounsfield units (HU). Hyperinflated tissue was defined as −1000 to −901 HU, normal aerated tissue as −900 to −501 HU, poorly aerated tissue (PAT) as −500 to −101 HU and non-aerated tissue (NAT) as −100 to +100 HU. Alveolar recruitment (RECALV) with PEEP or inspiration was defined as the decrease in poorly aerated and non-aerated lung parenchyma.
Statistical analysis
Clinical characteristics and ventilatory data are presented as means ± SD. Comparisons on the amount of gas and tissue with different levels of PEEP, and ventilatory and oxygenation data were evaluated by one-way analysis of variance (ANOVA). The level of significance was set at p less than 0.05.
Results
Ten patients (5 M, 5 F, 58±16 years) with ALI/ARDS (PaO2/FIO2 46–214) underwent a thoracic CT scan for diagnostic or therapeutic reasons. Clinical characteristics and baseline parameters are shown in Table 1. Figure 3 illustrates the end-expiratory basal CT sections taken at different PEEP levels in all patients.
Protocol 1
The ZEEP condition was associated with an increase in basal densities (Fig. 3) and a decrease in SatO2 in most patients. Increasing PEEP up to 40 cmH2O significantly increased lung volume, because of an increase in gas volume and no change in lung weight, even at high levels of PEEP (Fig. 4). Left lung volumes were significantly smaller than the right ones at all PEEP levels (Fig. 4). A caudal displacement of the lung during the LRM, especially at high levels of PEEP, was seen in all patients (Fig 3). Patient 5 presented severe desaturation at ZEEP despite FIO2 1.0, which was resolved after several LRMs. No severe hypotension was observed in any patient.
Gas volume (left panel) and weight (right panel) for the right lung (dashed squares) and left lung (open squares) basal CT sections during the first protocol (patients 1 to 5). Increments in positive end-expiratory pressure (PEEP) increased the amount of gas volume in all patients without changing the weight. Data are means ± SD. *p<0.05 versus baseline value
Protocol 2
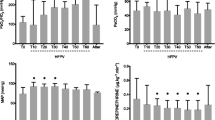
The increase in PEEP level from 10 to 30 cmH2O was associated with an increase in lung volume, due to an increase in gas volume (Fig. 5). The increase in gas volume between end-expiration and end-inspiration was greater at PEEP 10 cmH2O than at PEEP 30 cmH2O, despite the same driving pressure (20 cmH2O). PaO2, PaO2:FIO2 ratio and MAP increased with PEEP, while Vt and total Cst decreased (Table 2).
Volume of gas of the left and right lung basal CT sections, respectively, during the second protocol (patients 6 to 10). During the lung recruitment maneuver (LRM), positive end-expiratory pressure (PEEP) up to 30 cmH2O increased the amount of gas at end-expiration (closed squares) and end-inspiration (open squares). Five minutes after the LRM (10-post), gas volume at end-expiration and end-inspiration is greater, but not significantly, than baseline values (10), despite similar airway pressures. Data are means ± SD. *p<0.05 versus baseline value
The end-expiration basal CT section volume at PEEP 30 cmH2O was far greater than end-inspiration basal CT section volume at PEEP 10 cmH2O, despite similar static airway pressures, this is 30 cmH2O (Fig. 5).
Three to five minutes after the LRM, there was a significant improvement in oxygenation index compared to baseline values. However, basal CT section lung volumes at end-expiration and end-inspiration were higher, but not significantly so, than baseline values. There were also non-significant improvements in lung mechanics and PaO2 and PaO2:FIO2 ratio (Fig 5 and Table 2).
Alveolar recruitment and hyperinflation
Poorly aerated and non-aerated tissue accounted for 60.0±29.1% of lung parenchyma at PEEP 10 cmH2O, while only 1.1±1.8% was hyperinflated for all ten patients (Fig. 6). Alveolar recruitment at end-expiration with PEEP 20 and 30 cmH2O, compared to PEEP 10 cmH2O, reached 16.2±28.0% and 33.4±13.8%, respectively (p<0.05). This was mainly due to an absolute decrease of non-aerated zones, while poorly distended zones tended to remain constant (Fig. 6).
Histogram of lung tissue (ml) during the second protocol (patients 6 to 10). Normal aerated tissue at end-expiration (exp.) increased due to alveolar recruitment (decrease in poorly and non-aerated tissue) when increasing positive end-expiratory pressure (PEEP) levels, with further increases at end-inspiration (insp.). Hyperinflated tissue increased to a lesser degree, both when increasing PEEP levels and during inspiration
Inspiration in patients 6 to 10 further increased alveolar recruitment compared to end-expiration values at all PEEP levels. However, this increment was lower with increasing PEEP levels, from 22.0±7.3% to 15.7±4.5% and 11.1±3.3% at PEEP 10, 20 and 30 cmH2O, respectively (Fig. 6). Hyperinflation in patients 6 to 10 increased slightly up to 1.9±2.8 and 2.9±4.0% with PEEP 20 and 30 cmH2O, respectively (p<0.05), and to a lesser degree with inspiration (Fig. 6).
Discussion
This study shows that LRMs using high levels of PEEP improve oxygenation by expanding previously collapsed alveoli without inducing too much hyperinflation in patients with acute respiratory failure. On the other hand, a LRM during the CT scan seems safe and gives morphologic and functional information that could be useful in setting ventilatory parameters.
Alveolar recruitment
Lung recruiting maneuvers should be considered as high distending airway pressures for short periods of time. They can improve ventilation at low Vt and/or airway pressures by expanding collapsed alveoli [17, 18, 19]. Although we did not register hemodynamic parameters, some clinical data have proved it to be safe. Recently, Lapinsky et al. tried a sustained inflation pressure of 30–45 cmH2O for up to 20 s in 14 hypoxemic patients [20]. Significant improvement in oxygenation occurred in the majority of patients within 10 min without barotrauma. There was a reversion to mild hypotension immediately after the maneuver was stopped. Sighs up to 45 cmH2O plateau pressure have also been used and have shown marked improvement in oxygenation and lung elastance [19]. Recently, Grasso et al. applied 40 cmH2O of continuous positive airway pressure for 40 s in 22 ARDS patients during use of the ARDS Net lung-protective ventilatory strategy, and observed a 50% increase in PaO2:FIO2 ratio in half of them [22]. The other half, in whom PaO2:FIO2 increased by only 20%, had been ventilated for a longer period of time and showed a significant decrease in cardiac index and mean arterial pressure.
Our study shows that alveolar recruitment is the main mechanism for the increase in lung volume during our LRM, which is clearly noted on visual inspection and quantified through voxel density analysis. The decrease in non-aerated tissue accounts for most of the increase in normal aerated tissue, as also shown by other studies [1, 7].
There has been controversy about how to assess alveolar recruitment and two basic CT approaches have been described in the literature [1, 7, 26, 27]. While Rouby et al. have measured the whole lung under two conditions (0 and 10 or 15 cmH2O PEEP and FIO2 1.0), Gattinoni et al. have used one to three slices with different inspiratory and expiratory airway pressures. We used the one CT slice technique, which sometimes is not representative of the entire lung, but allows study of the behavior of the lung at several airway pressures, under less X-ray exposure [1, 7, 27].
Most importantly, as shown recently by Crotti, our study reaffirms that alveolar recruitment is a pan-inspiratory phenomenon [27]. This is clearly shown by the increase in normal aerated tissue during inspiration at all PEEP levels. This simple fact leads to several conclusions. Firstly, as suggested by other authors, PEEP above the inflection point does not predict full recruitment [28, 29, 30]. Secondly, end-inspiratory volume depends on peak inspiratory pressures [1, 27]. In other words, PEEP prevents the closure of alveoli previously opened by inspiration [1, 29, 30].
Hyperinflation and overstretching
Although LRM recruits alveolar units, it also produces hyperinflation and, to a greater degree, overstretching. These two terms have been recently introduced to be used instead of overdistention, which had been previously used to describe these two phenomena imprecisely [1]. Hyperinflation was quantified by the increase in densities below −900 HU with increasing PEEP levels and inspiration. In contrast, overstretching—a mechanical concept—is shown by the decrease in Vt at the same driving pressures (Table 2) and the lower gas volume change between end-expiration and end-inspiration at 10 and 30 cmH2O PEEP (Fig. 5).
Our data are similar to other studies that have shown, despite different definitions, that only a small fraction of the lung parenchyma is hyperinflated even with high levels of PEEP [1, 8, 31]. However, it is noteworthy that a small fraction of hyperinflated tissue can have such an impact on lung mechanics. At least two explanations can be forwarded. First, as our voxel size equals hundreds of alveoli, voxels including a heterogeneous population will be read as their average density. Alveoli at the extremes of density, such as hyperinflated ones, will have less opportunity to be detected [1, 26, 32, 33]. If this is the case, the real amount of hyperinflated tissue may be underestimated. On the other hand, overstretching not only occurs in hyperinflated alveoli but has also been described at the boundaries of atelectatic and normal tissue [34, 35]. This will clearly not be detected on density analysis as hyperinflated, but most probably as poorly aerated, tissue. Thus, overstretching will result from the amount of both atelectatic and hyperinflated lung parenchyma. The real interaction between hyperinflated and overstretched lung tissue deserves more experimental and clinical studies.
Usefulness of lung recruitment maneuver during computed tomography scan
Computed tomography scan has been very useful in understanding lung mechanics and ARDS pathophysiology. First, it has shown that ARDS involves not a rigid lung but a highly heterogeneous entity that works as a smaller lung [3]. Moreover, Cst and Qs/Qt are related to the amount of gas and tissue, respectively, suggesting a relationship between CT findings and lung mechanics and gas exchange. More recently, it has been shown that the lung works as a sponge, which was clearly seen in some patients in our study [4, 5, 7].
At the very beginning of our study, we wanted to assess CT scan under different pressure levels so as to set the best ventilatory strategy. Unfortunately, as the lung moves in different directions, it is impossible to assess the three dimensional movement of the thorax with a single CT slice [1, 26, 36]. Despite the beautiful images obtained by this method, we were not able to measure the downward movement of the lung. Secondly, and as many authors have noted, loss of recruitment can be more dangerous than overdistention [14, 15 19]. Some ARDS patients present severe desaturation on ZEEP, which is clearly detrimental, so after the first five patients in the present study we did not include the ZEEP condition in the following CT protocols.
However, CT images give additional information to that which could be obtained at the bedside [37]. Pleural effusions and barotrauma, which can go undetected on chest X-rays are easily recognized and can be drained promptly. The macroscopic behavior of the lung parenchyma with positive pressure ventilation may also orient ventilation beyond numerical data. For instance, differential ventilation was applied in patient number 2 as recruitment in the left lung started at the 20–25 cmH2O PEEP level. Patients 5 and 6, in contrast, had the typical sponge lung and high PEEP levels were used.
Most important, as mentioned before, is the relationship between expiration and inspiration. Mechanical ventilation is not a static process but a highly dynamic one. Static P-V curves depict the mechanical status of the lung at rest and not during the ventilatory cycle [22, 29]. In contrast, CT-generated images provide a real time image of the dynamic performance of the lung at end-inspiration and end-expiration (Fig. 7). This has been tried, but not accomplished, with expiratory and inspiratory P-V curves [22]. We did not perform expiratory P-V curves, which could have shown a better relationship with CT findings [22, 29]. Dynamic CT images could have a great impact on setting ventilatory parameters, namely PEEP and Vt (or alveolar distending pressures), by improving alveolar recruitment and limiting overstretching [1, 37]. However, patient transfer, X-ray exposure and complex measurements at the present time preclude the clinical use of CT for optimizing ventilatory management at the bedside.
Pressure-volume curve obtained from data from Table 2 and Fig. 5. At the same airway pressures (30 cmH2O), two lung volumes are obtained depending on their position on the ventilatory cycle. A hypothetical inspiratory and expiratory pressure-volume curve (dashed line) is traced (FRC functional residual capacity)
In conclusion, LRMs effectively improve oxygenation and lung mechanics, mainly by expanding previously collapsed alveoli. Our study adds new information on the mechanisms involved in the use of high airway pressures for short periods of time. The way in which LRMs should be employed in protective strategies still needs to be explored.
Dynamic lung CT images could play an important role in improving alveolar recruitment while avoiding overstretching. Fast and friendly software connecting CT machines and ventilators could bring this into clinical practice.
References
Gattinoni L, Caironi P, Pelosi P, Goodman LR (2001) What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med 164:1701–1711
Brismar B, Hedenstierna G, Lundquist H, Strandberg A, Svensson L, Tokics L (1985) Pulmonary densities during anesthesia with muscular relaxation—a proposal of atelectasis. Anesthesiology 62:422–428
Gattinoni L, Pesenti A, Bombino M, Baglioni S, Rivolta M, Rossi F, Rossi G, Fumagalli R, Marcolin R, Mascheroni D et al. (1988) Relationships between lung computed tomographic density, gas exchange and PEEP in acute respiratory failure. Anesthesiology 69:824–832
Gattinoni L, Pelosi P, Vitale G, Pesenti A, D'Andrea L, Mascheroni D (1991) Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure. Anesthesiology 74:15–23
Gattinoni L, D'Andrea L, Pelosi P, Vitale G, Pesenti A, Fumagalli R (1993) Regional effects and mechanism of positive end-expiratory pressure in early adult respiratory distress syndrome. JAMA 269:2122–2127
Gattinoni L, Bombino M, Pelosi P, Lissoni A, Pesenti A, Fumagalli R, Tagliabue M (1994) Lung structure and function in different stages of severe adult respiratory distress syndrome. JAMA 271:1772–1779
Gattinoni L, Pelosi P, Crotti S, Valenza F (1995). Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med 151:1807–1814
Vieira SR, Puybasset L, Richecoeur J, Lu Q, Cluzel P, Gusman PB, Coriat P, Rouby JJ (1998) A lung computed tomographic assessment of positive end-expiratory pressure-induced lung overdistension. Am J Respir Crit Care Med 158:1571–1577
Puybasset L, Cluzel P, Chao N, Slutsky AS, Coriat P, Rouby JJ (1998) A computed tomography scan assessment of regional lung volume in acute lung injury. The CT Scan ARDS Study Group. Am J Respir Crit Care Med 158:1644–1655
Puybasset L, Cluzel P, Gusman P, Grenier P, Preteux F, Rouby JJ (2000) Regional distribution of gas and tissue in acute respiratory distress syndrome. I. Consequences for lung morphology. CT Scan ARDS Study Group. Intensive Care Med 26:857–869
Rouby JJ, Puybasset L, Cluzel P, Richecoeur J, Lu Q, Grenier P (2000) Regional distribution of gas and tissue in acute respiratory distress syndrome. II. Physiological correlations and definition of an ARDS Severity Score. CT Scan ARDS Study Group. Intensive Care Med 26:1046–1056
Puybasset L, Gusman P, Muller JC, Cluzel P, Coriat P, Rouby JJ (2000) Regional distribution of gas and tissue in acute respiratory distress syndrome. III. Consequences for the effects of positive end-expiratory pressure. CT Scan ARDS Study Group. Adult Respiratory Distress Syndrome. Intensive Care Med 26:1215–1227
Lachmann B (1992) Open up the lung and keep the lung open. Intensive Care Med 18:319–321
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282:54–61
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Richard JC, Maggiore SM, Jonson B, Mancebo J, Lemaire F, Brochard L (2001) Influence of tidal volume on alveolar recruitment. Respective role of PEEP and a recruitment maneuver. Am J Respir Crit Care Med 163:1609–1613
Foti G, Cereda M, Sparacino ME, De Marchi L, Villa F, Pesenti A (2000). Effects of periodic lung recruitment maneuvers on gas exchange and respiratory mechanics in mechanically ventilated acute respiratory distress syndrome (ARDS) patients. Intensive Care Med 26:501–507
Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, Lissoni A, Gattinoni L (1999) Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med 159:872–880
Lapinsky SE, Aubin M, Mehta S, Boiteau P, Slutsky AS (1999) Safety and efficacy of a sustained inflation for alveolar recruitment in adults with respiratory failure. Intensive Care Med 25:1297–1301
Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, Slutsky AS, Ranieri VM (2002) Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology 96:795–802
Marini JJ, Amato MB (1998) Lung recruitment during ARDS. In: Marini JJ, Evans TW (eds) Acute Lung Injury. Springer Verlag, Berlin Heidelberg, pp 236–257
Monchi M, Bellenfant F, Cariou A (1998) Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med 158:1076–1081
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R (1994) Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med 20:225–232
Levy P, Smilowski T, Corbeil C, Albala M, Pariente R, Milic-Emili J, Jonson B (1989) A method for studying the static volume-pressure curves of the respiratory system during mechanical ventilation. J Crit Care 4:83–89
Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ (2001) Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1444–1450
Crotti S, Mascheroni D, Caironi P, Pelosi P, Ronzoni G, Mondino M, Marini JJ, Gattinoni L (2001) Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med 164:131–140
Hickling KG (1998) The pressure-volume curve is greatly modified by recruitment. A mathematical model of ARDS lungs. Am J Respir Crit Care Med 158:194–202
Ranieri VM, Tortorella C, Grasso S (1998) Pressure-volume relationship in the injured lung. In: Marini JJ, Evans TW (eds) Acute Lung Injury. Springer Verlag, Berlin Heidelberg, pp 213–235
Rimensberger PC, Cox PN, Frndova H, Bryan AC (1999) The open lung during small tidal volume ventilation: concepts of recruitment and "optimal" positive end-expiratory pressure. Crit Care Med 27:1946–1952
Dambrosio M, Roupie E, Mollet JJ, Anglade MC, Vasile N, Lemaire F, Brochard L (1997) Effects of positive end-expiratory pressure and different tidal volumes on alveolar recruitment and hyperinflation. Anesthesiology 87:495–503
Drummond GB (1998) Computed tomography and pulmonary measurements. Br J Anaesth 80:665–671
Brochard L (2001) Watching what PEEP really does. Am J Respir Crit Care Med 163:1291–1292
Marini JJ (2001) Recruitment maneuvers to achieve an "open lung"—whether and how? Crit Care Med 29:1647–1648
Marini JJ (1996) Tidal volume, PEEP and barotrauma. An open and shut case? Chest 109:302–304
Lu Q, Malbouisson LM, Mourgeon E, Goldstein I, Coriat P, Rouby JJ (2001) Assessment of PEEP-induced reopening of collapsed lung regions in acute lung injury: are one or three CT sections representative of the entire lung? Intensive Care Med 27:1504–1510
Pesenti A, Tagliabue P, Patroniti N, Fumagalli R (2001). Computerised tomography scan imaging in acute respiratory distress syndrome. Intensive Care Med 27:631–639
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the XIII Annual Congress of the European Society of Intensive Care Medicine, Rome, 2000.
Rights and permissions
About this article
Cite this article
Bugedo, G., Bruhn, A., Hernández, G. et al. Lung computed tomography during a lung recruitment maneuver in patients with acute lung injury. Intensive Care Med 29, 218–225 (2003). https://doi.org/10.1007/s00134-002-1618-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-002-1618-6