Abstract
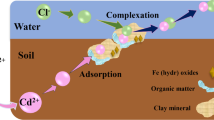
The present study examined Cd solubility in pH 2–12 fresh and seawater media with and without humin to determine Cd species composition. The study, based on the Langmuir–Hinshelwood kinetics model, was conducted to determine the kinetic parameters of Cd(II) adsorption onto humin. We employed the Langmuir and Freundlich models to derive thermodynamic parameters. Aquo (Cd(H2O) 2+6 ) and chloro- (CdCl+ and CdCl2) complexes were responsible for Cd(II) adsorption onto humin. Results showed Cd as Cd(II) and Cd(H2O) +26 was water soluble at 2 < pH < 7; with a portion of the soluble Cd precipitating as Cd(OH)2. The Cd(II) rate constant (k) in freshwater was 0.5 × 10−3 (min−1), occurring in a single phase, while in seawater fast and slow phase values for k were 31.88 × 10−3 and 6.2 × 10−3 (min−1), respectively. The adsorption curves showed a better fit with the Langmuir than the Freundlich model.




Similar content being viewed by others
References
Alvarez-Puebla RA, Valenzuela-Calahorro C, Garrido JJ (2004) Modeling the adsorption and precipitation processes of Cu(II) on humin. J Colloid Interface Sci 277:55–61
Cerqueira Sda CA, Romao LPC, Lucas SCO, Fraya LE, Simoes ML, Hammer P, Lead JR, Mangoni AP, Mangrich AS (2012) Spectroscopic characterization of the reduction and removal of chromium(VI) by tropical peat and humin. Fuel 91:141–146
Dader M, Colilla M, Hitzky ER (2003) Biopolymer—clay nanocomposites based on chitosan intercalated in montmorillonite. Chem Mater 15:3774–3780
Gafney SJ, Marley NA, Clark SB (1996) Humus, fulvic acid, and organic colloidal material in the environment. In: Gaffney JS et al (eds) Humic and fulvic acid: isolation, structure, and environmental role. American Chemical Society, Washington DC
Gauthier TD, Seltz WR, Grant CL (1987) Effect of structural and compositional variation of dissolved humic materials on pyrene Koc values. Environ Sci Technol 21:243–248
Huheey JE, Keiter EA, Keiter RL (1993) Inorganic chemistry, Ed 4th, Harper Collins College Publisher, California, CA, USA
Jain AK, Gupta VK, Jain S, Suhas (2004) Removal of chlorophenols using industrial wastes. Environ Sci Technol 38:1195
Jiang J, Kappler A (2008) Kinetics of microbial and chemical reduction of humic substances: implication for electron shuttling. Environ Sci Technol 42(10):3563–3569
Marschner B, Winkler R, Jodemann D (2005) Factors controlling the partitioning of pyrene to dissolved organic matter extracted from different soil. Eur J Soil Sci 56:299–306
Rice JA (2001) Humin. Soil Sci 166:848–857
Rice JA, MacCarthy P (1990) A model of humin. Environ Sci Technol 24(12):1875–1977
Santosa SJ, Narsito Lesbani A (2001) The determination of active site, capacity, energy and rate constant on the adsorption of Zn(II) and Cd(II) on chitin. J Ion Exch 14:89–92
Schnoor JL (1996) Environmental modeling. John Wiley & Sons Inc, New York
Simson MJ (2006) Nuclear magnetic resonance based investigation of contaminant interactions with soil organic matter. Soil Sci Soc Am J 70:995–1004
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions. John Wiley & Sons Inc., New York
Wen B, Zhang JJ, Zhang SZ, Shan XQ, Khan SU, Xing B (2007) Phenanthrene sorption to soil humic acid and different humin fractions. Environ Sci Technol 41:3165–3171
Xing B (2001) Sorption of anthropogenic organic compounds by soil organic matter: a mechanistic consideration. Can J Soil Sci 81:317–323
Yu Z, Sharma S, Huang W (2006) Differential roles of humic acid and particulate organic matter in the equilibrium sorption of atrazine by soil. Environ Toxicol Chem 25:1975–1983
Zhang J, Wang S, Wang Q, Wang N, Li Cuilan, Wang L (2013) First determination of Cu adsorption on soil humin. Environ Chem Lett 11:41–46
Acknowledgments
The research was financially supported by a grant-in-aid for Scientific Research (No. 25550011 and 25110505) from the Ministry of Education, Science, Sports, and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andreas, R., Zhang, J. Characteristics of Adsorption Interactions of Cadmium(II) onto Humin from Peat Soil in Freshwater and Seawater Media. Bull Environ Contam Toxicol 92, 352–357 (2014). https://doi.org/10.1007/s00128-014-1205-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-014-1205-x