Abstract
Aims/hypothesis
The prevalence of gestational diabetes mellitus (GDM) is increasing worldwide in all ethnic groups. Low vitamin B12 and low/high folate levels may contribute to GDM risk, but there is conflicting evidence. Our aim is to assess the relationships of early pregnancy vitamin B12 and folate levels with the risk of GDM status at 26–28 weeks of gestation.
Methods
This was a prospective, multi-centre, multi-ethnic cohort study (n = 4746) in the UK. Participants who were eligible to be selectively screened as per the National Institute for Health and Care Excellence (NICE) criteria were included in the study.
Results
GDM prevalence was 12.5% by NICE and 14.7% by International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria. Folate deficiency (1.3%) was rare but B12 insufficiency (42.3% at <220 pmol/l) and folate excess (36.5%) were common in early pregnancy. Early pregnancy median B12 levels were lower, and folate levels higher, in women who were diagnosed with GDM at 26–28 weeks. B12 was negatively associated with fasting plasma glucose (1 SD: −0.06 mmol/l; 95% CI −0.04, −0.08; p < 0.0001) and 2 h plasma glucose levels (−0.07 mmol/l; 95% CI −0.02, −0.12; p = 0.004). Higher B12 was associated with 14.4% lower RR of IADPSG-GDM (0.856; 95% CI 0.786, 0.933; p = 0.0004) after adjusting for key confounders (age, parity, smoking status, ethnicity, family history, household income and folate status). Approximately half of this association was mediated through BMI. Folate was positively associated with 2 h plasma glucose levels (0.08 mmol/l; 95% CI 0.04, 0.13; p = 0.0005) but its relationship with fasting plasma glucose was U-shaped (quadratic β: 0.011; p = 0.05). Higher folate was associated with 11% higher RR of IADPSG-GDM (adjusted RR 1.11; 95% CI 1.036, 1.182; p = 0.002) (age, parity, smoking status, ethnicity, family history, household income and B12 status). Although no interactions were observed for B12 and folate (as continuous variables) with glucose levels and GDM risk, a low B12–high folate combination was associated with higher blood glucose level and risk of IADPSG-GDM (adjusted RR 1.742; 95% CI 1.226, 2.437; p = 0.003).
Conclusions/interpretation
B12 insufficiency and folate excess were common in early pregnancy. Low B12 and high folate levels in early pregnancy were associated with small but statistically significant changes in maternal blood glucose level and higher RR of GDM. Our findings warrant additional studies on the role of unmetabolised folic acid in glucose metabolism and investigating the effect of optimising early pregnancy or pre-conception B12 and folate levels on subsequent hyperglycaemia.
Trial registration:
ClinicalTrials.gov NCT03008824.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Gestational diabetes mellitus (GDM) is a common medical disorder in pregnancy, estimated to affect more than 20 million pregnancies worldwide, and causes significant short- and long-term consequences to both the women and their offspring [1]. While management of GDM reduces the short-term complications, it does not abolish them completely [2, 3]. In addition, adverse effects for the offspring may have already happened prior to the diagnosis of GDM [4,5,6]. Therefore, prevention of GDM would be a better approach to reduce adverse outcomes in pregnant women and their offspring. This will require identifying and modifying risk factors before the diagnosis of GDM. However, most prevention studies in early pregnancy (such as diet and lifestyle interventions) have focused on a single modifiable risk factor, obesity, with limited success. Identification of other modifiable risk factors that contribute to GDM risk is needed.
Folate and B12 are essential micronutrients for the metabolism of single carbon atoms (known as 1-C metabolism) and these pathways are involved in DNA methylation and synthesis of amino acids, nucleic acids and lipids [7, 8]. High total homocysteine (tHcy) is a marker of deficiency of either nutrient as B12 is a coenzyme for the folate-dependent methylation of tHcy to methionine. In addition, B12 is a coenzyme for the action of methyl malonyl-CoA mutase in the mitochondria, involved in the conversion of methyl malonyl-CoA to succinyl-CoA, and degradation of odd-chain fatty acids and branched-chain amino acids (BCAAs) [7, 8]. Altered levels of BCAAs have been shown to precede the onset of hyperglycaemia and type 2 diabetes [9]. Folate and B12 thus play a crucial and inter-dependent role in genomic stability, methylation potential, epigenetics, carbohydrate metabolism, fatty acid β-oxidation and protein synthesis [7, 8]. Some of these mechanisms contribute to glucose homeostasis as well as adverse metabolic programming of the offspring [10,11,12].
Conflicting data have been reported regarding associations of B12 and folate with the risk of GDM. Most of the studies measured these B vitamins at the time of diagnosis of GDM in late pregnancy or used estimated folate intake [13,14,15,16,17,18,19,20,21]. Higher tHcy has been linked to impaired endothelial function, with resulting reduction in insulin sensitivity, outside pregnancy [22], but its role in glucose metabolism in pregnancy is not clear. Previous studies showed positive, negative or no associations with the risk of GDM [23,24,25,26]. The purpose of our study is to examine the relationships of B12, folate and tHcy levels in early pregnancy with the glucose levels and risk of GDM in late pregnancy, in a large, multi-ethnic, prospective cohort study.
Methods
Participant selection and recruitment
Pregnant women attending antenatal care in ten study sites across the UK were recruited between 2012 and 2018 to the ‘Micronutrients in Pregnancy as a Risk Factor for Gestational Diabetes and Effects on Mother and Baby’ (PRiDE) study (clinicaltrials.gov number: NCT03008824). Ethical approval was obtained from the National Research Ethics Committee (12/WM/0010). All participants provided written informed consent. The detailed study protocol, including all the standard operating procedures, is available at the departmental website, University of Warwick (https://warwick.ac.uk/fac/sci/med/staff/saravanan/pride_study_protocol.pdf). In brief, pregnant women aged between 18 and 45 years who were at less than 16 weeks of gestation and fulfilled the National Institute for Health and Care Excellence (NICE) criteria for screening for GDM were included [27]. All centres followed the NICE screening guidelines (at least one of the following risk factors): BMI ≥30 kg/m2; previous GDM; previous unexplained stillbirth or birthweight ≥4.5 kg; first degree relative with diabetes; ethnic minority group. Two centres screened additional women who were aged ≥35 years at booking or had a history of polycystic ovarian syndrome. The exclusion criteria were: pre-gestational diabetes mellitus; a previous pregnancy with a neural tube defect; multiple gestation; severe anaemia (haemoglobin <10 g/dl); confirmed vitamin B12 or folate deficiency or having received B12 injections within the last 6 months.
Data collection
Participants’ demographic information including their marital, employment and smoking status; educational attainment; household income; and medical, obstetric and supplement intake (type and duration) history were collected during the screening visit. Their height, weight, BMI and waist circumference were measured at the booking and OGTT visits. A random blood sample at booking and fasting and 2 h blood samples at OGTT using 75 g of anhydrous glucose after an overnight fast of at least 10 h were taken. As the focus of this analysis is the diagnosis of GDM, birth outcomes data are not reported here.
Biochemical analysis
Blood samples were kept in refrigerated containers, centrifuged within 30 min of collection and stored in −80°C freezers until analysis, except for glucose which was done immediately. Plasma glucose was determined by the hexokinase enzymatic method using a Synchron CX7 auto-analyser (Beckman Coulter, Fullerton, CA, USA). Serum B12 and folate were measured by electro-chemiluminescent immunoassay (Roche Cobas analyser, Roche Diagnostics, Burgess Hill, UK). The intra- and inter-assay CVs for B12 and folate were 2.0% and 3.1%; and 3.1% and 3.8%, respectively. Plasma tHcy was determined by stable isotopic dilution analysis using a Shimadzu HPLC system with an auto-sampler coupled to the detection system of an API 6500 QTrap tandem mass spectrometer (liquid chromatography mass spectrometry [LCMS]) (Applied Biosystems, Warrington, UK) [28]. A calibration curve and quality control samples (low, medium and high concentrations within the limits of quantification; Waters, UK) were set up for each sample batch that was analysed. Inter- and intra-assay CVs for tHcy were 7.0% and 8.1%. This was comparable to an assay used by a regional laboratory in Birmingham [29] and below the recommended CV thresholds [30].
Definitions
Ethnicity was coded using standard definitions. ‘Other’ ethnic group included North African, Black African, Caribbean, Asian, South East Asian, Middle Eastern and mixed ethnicity. GDM diagnoses based on NICE (NICE-GDM; fasting plasma glucose [FPG] ≥5.6 mmol/l or 2 h post-load plasma glucose [2 h-PG] ≥7.8 mmol/l) and International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria (IADPSG-GDM; FPG ≥5.1 mmol/l or 2 h-PG ≥8.5 mmol/l) were recorded [27, 31]. B12 insufficiency was defined using two different cut-offs: <150 pmol/l and <220 pmol/l. The former is commonly used to define B12 deficiency in adults and in pregnancy, while others have suggested the higher cut-off in pregnancy, as this was associated with low birthweight and elevated tHcy and methylmalonic acid (MMA) levels [32,33,34]. Folate deficiency was defined as <10 nmol/l and folate excess as >45 nmol/l [35].
Pre-defined outcomes
Primary
Differences in the risk of GDM in women with and without early pregnancy B12 insufficiency.
Secondary
-
1.
Associations of B12 and folate with blood glucose level/GDM risk (B12, folate, FPG and 2h-PG as continuous variables);
-
2.
Association between tHcy and blood glucose level/GDM risk;
-
3.
Association between ‘low B12–high folate’ status and blood glucose level/GDM risk; and
-
4.
Ethnic differences in associations of B12, folate and tHcy with blood glucose level/GDM risk.
-
5.
We also aimed to assess the role of BMI in these associations.
Statistical analysis
Sample size calculations
There are no published data on the rate of first trimester B12 insufficiency in GDM women in the UK. At study design, preliminary results from our group showed 15% insufficiency (<150 pmol/l) in early pregnancy and 10–12% prevalence of GDM [27]. To detect a 5% difference in the prevalence of GDM between normal and B12 insufficiency with 90% power at the 5% significance level, 3822–4322 women were required; we aimed to recruit 4500 women in early pregnancy, allowing for a 10% drop-out rate.
Analyses
Women’s characteristics were reported using appropriate descriptive statistics (mean and SD) or median and IQR for continuous variables and percentages for categorical variables. χ2 and t tests were used to assess differences in categorical and continuous variables, respectively. To define participants’ socioeconomic status (SES), we considered their occupation and total household income. For the main outcome analyses, household income was used as the main measure of SES, because this is directly associated with higher purchase and intake of vegetables and fruit which in turn correlate with higher folate status [36].
RR and 95% CI for GDM in relation to different micronutrients were estimated using multiple log-binomial regression models. Multiple linear regression analyses were used to test the associations of early pregnancy micronutrients with FPG and 2h-PG at OGTT. The micronutrients were standardised, by subtracting the mean and dividing by SD, to aid comparison. To further understand the shapes of the relationships of FPG and 2h-PG at OGTT with B12 and folate, cubic spline regression models were fitted. Ethnic-specific associations of the micronutrients with glucose levels and the risk of GDM were tested using similar regression models.
From published studies, while the causal direction is clear between BMI and glucose, this is not clear between BMI and B12 and folate. Therefore, to understand this complex interplay, two different models were used, a priori, for all the regression analyses while adjusting for possible confounders. Model 1 included age, parity, smoking status, ethnicity, family history, household income and respective micronutrient status (B12 for folate, folate for B12, and B12 and folate for tHcy). Model 2 included model 1 plus BMI. Other possible confounders such as marital status and gestational weight gain were not used as they did not contribute to the exploratory and/or outcome variables. Effects of interaction between B12 and folate on blood glucose level and the risk of GDM were investigated both as continuous variables and after categorising them by tertiles. The first tertile of folate and the third tertile of B12 were used as the reference tertiles.
As the proportion of missing data was low for B12, folate and tHcy (maximum 2.6%), the main analysis reported excluded these patients. All analyses were implemented using R version 4.0.0 [37].
Results
A total of 4746 eligible pregnant women were recruited. Their mean gestational age was 12.5 ± 1.4 weeks (Fig. 1). Ethnicity proportions were similar at both the booking and OGTT visits (Table 1, Fig. 1). B12 insufficiency (42.3%) and folate excess (36.5%) were common but folate deficiency (1.3%) was rare in all ethnic groups. BMI was inversely associated with B12 and positively associated with folate and tHcy (electronic supplementary material [ESM] Table 1). Folic acid and multivitamin supplement usage were associated with higher folate and B12 levels, respectively, and lower tHcy. While household income was unrelated to B12 levels, higher income was associated with higher folate and lower tHcy levels.
Obesity was the most common reason for selective screening in white women, and all ethnic minority women were universally screened as per NICE guidelines (ESM Table 2). A total of 538 (12.5%) women had NICE-GDM and 633 (14.7%) had IADPSG-GDM. The characteristics of study participants by GDM status are shown in Table 2 (IADPSG-GDM). For simplicity, all the NICE-GDM data are presented in the ESM (ESM Table 3, ESM Fig. 1). Both folic acid and multivitamin supplement usage were similar between GDM and non-GDM groups.
Primary outcome: B12 insufficiency and GDM
Early pregnancy median B12 levels were significantly lower in women who were later diagnosed to have IADPSG-GDM (Table 2). B12 insufficiency at <220 pmol/l was associated with 38.3% higher adjusted RR (aRR) of IADPSG-GDM (aRR 1.383; 95% CI 1.157, 1.652; p = 0.0004) in model 1 and 20.3% higher in model 2 (aRR 1.203; 95% CI 1.003, 1.443; p = 0.05). The associations with NICE-GDM and with B12 insufficiency at <150 pmol/l are shown in ESM Table 4.
Secondary outcomes
B12, blood glucose level and GDM
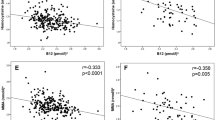
The cubic spline graphs showed that the relationships between B12 and glucose levels were linear (Fig. 2a,b) and B12 was negatively associated with FPG, 2h-PG and risk of GDM (Figs 3a,b, 4a). A 1 SD increase in B12 (116 pmol/l) was associated with a 0.06 mmol/l lower FPG (95% CI −0.04, −0.08; p < 0.0001), a 0.07 mmol/l lower 2h-PG (95% CI −0.02, −0.12; p = 0.004) and a 14.4% lower RR of IADPSG-GDM (0.856; 95% CI 0.786, 0.933; p = 0.0004; model 1), after adjusting for key confounders. Effect sizes halved when adjusted for BMI (model 2; Figs 3a,b, 4a).
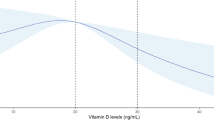
(a–d) Cubic spline regression analysis of the relationship of early pregnancy B12 and folate with fasting and 2 h glucose levels at OGTT. (a) The relationship between B12 and fasting glucose and (b) 2 h glucose and (c) the relationship between folate and fasting glucose and (d) 2 h glucose using the cubic spline regression analyses. The regression curve is shown in bold, and the shaded area represents the 95% confidence band. The dotted vertical lines represent the reference range for the B12 (150, 220 and 660 pmol/l) and folate levels (10 and 45 nmol/l)
(a–d) Associations of early pregnancy B12/folate levels with fasting and 2 h glucose levels. (a) The standardised β coefficient of the associations of early pregnancy B12 levels (as a continuum) with fasting glucose at OGTT and (b) with 2 h glucose levels. (c) The standardised β coefficient of the associations of early pregnancy folate levels (as a continuum) with fasting glucose at OGTT and (d) with 2 h glucose levels. Data are shown with 95% CI. Two models are shown for each ethnic group. Circles depict model 1 and triangles depict model 2. Model 1 is adjusted for the following covariates: age, parity, family history, household income, smoking and folate. Model 2 is adjusted for model 1 + BMI
(a, b) Early pregnancy B12/folate levels and risk of GDM. (a) The B12 and (b) folate levels in early pregnancy and aRR of IADPSG-GDM. Data are shown with 95% CI. Two models are shown for each ethnic group. Circles depict model 1 and triangles depict model 2. Model 1 is adjusted for the following covariates: age, parity, family history, household income, smoking and B12. Model 2 is adjusted for model 1 + BMI
Folate, blood glucose level and GDM
Folate was positively associated with blood glucose level, but its associations were complex. The cubic spline graphs show the different shapes of these relationships. With FPG, the relationship was U-shaped (quadratic β: 0.011; p = 0.05) and with 2h-PG it was linear and significant (Fig. 2c,d). A 1 SD increase in folate (12.16 nmol/l) was associated with 0.08 mmol/l higher 2 h-PG (95% CI 0.04, 0.13; p = 0.0005) and 11% higher RR of IADPSG-GDM (aRR 1.11; 95% CI 1.036, 1.182; p = 0.002) (model 1; Figs 3c,d, 4b). Similar to B12, its effect size reduced when adjusted for BMI (model 2; Figs 3c,d, 4b). A sensitivity analysis, stratified by whether women were taking folate supplements or not, showed similar results (data not shown).
tHcy, blood glucose level and GDM
tHcy was inversely associated with blood glucose level and risk of GDM. The associations strengthened when adjusted for BMI, suggesting that the tHcy effect on blood glucose level/GDM is independent of BMI, B12 and folate (ESM Table 5).
Low B12–high folate imbalance on blood glucose level and GDM
While no multiplicative interactions were observed between B12 and folate as continuous variables (interaction effects on FPG, 2 h-PG, NICE-GDM and IADPSG-GDM having p values of 0.47, 0.20, 0.83 and 0.49, respectively), significant interactions were observed when B12 and folate were categorised as tertiles with RR of NICE-GDM (ESM Table 6). The opposing associations of B12 and folate with glucose and GDM result in the highest risk being in women within the lowest B12 and highest folate tertiles (ESM Fig. 2a–d). Compared with the ‘B12 tertile3+folate tertile1’ group, the ‘B12 tertile1+folate tertile3’ group had higher levels of FPG (0.21 mmol/l; p = 0.0001) and 2 h-PG (0.57 mmol/l; p < 0.0001) and higher aRR of IADPSG-GDM (aRR 1.742; 95% CI 1.226, 2.437; p = 0.003).
Ethnic differences of B12, folate and tHcy in blood glucose level and GDM risk
The B12 associations seemed to be stronger in South Asians, with a 22% lower RR for IADPSG-GDM in model 1 (aRR 0.78; 95% CI 0.63, 0.947; p = 0.01) and 14.7% in model 2 (aRR 0.853; 95% CI 0.689, 1.037; p = 0.12) (Fig. 4a). The inverse association with FPG was seen in all ethnic groups, but, for 2h-PG, it was significant only in South Asians in model 2 (Fig. 3a,b). However, no significant differences were observed between ethnic groups when an interaction function was used between B12 and ethnicity. The folate association with 2h-PG seemed to be present in all ethnic groups, but, for the risk of GDM, it was statistically significant only in white people (Figs 3d, 4b). The tHcy associations with FPG, 2h-PG and GDM were present only in white people (data not shown).
Discussion
In this early pregnancy prospective cohort study, we found that B12 insufficiency and folate excess were common in the first trimester. B12 levels were lower and folate levels were higher in women who were subsequently diagnosed to have GDM, around 27 weeks of gestation. Lower B12 in early pregnancy was associated with higher fasting and 2 h glucose levels as well as a higher RR of GDM. Folate associations were opposite, and higher folate level was associated with higher 2h-PG and higher risk of GDM. Approximately half of the effect sizes were mediated by BMI. These associations of B12 and folate levels with maternal blood glucose level were clinically small (between 0.06 and 0.08 mmol/l per SD) but statistically significant. Although no interactions were observed for B12 and folate levels (as a continuum) with glucose and GDM, a combination of low B12 and high folate tertiles had stronger associations with hyperglycaemia and GDM than the individual B12/folate levels. Having a larger sample size with multiple ethnic groups enabled us to explore the complex associations of folate with FPG and 2h-PG, and to clarify previously inconsistent findings with greater confidence.
Higher FPG and risk of GDM at low B12 levels was present in all ethnic groups, but the inverse association with 2h-PG was present mainly in South Asians. Similar inverse relationships at the time of OGTT were observed for B12 with FPG and insulin resistance in White British women [16], and with GDM in Indians (living in India and Singapore) [14, 15] and Italians [25]. In contrast, a recent study showed a direct relationship between early pregnancy B12 and 1 h and 2 h plasma glucose levels in a Chinese population. However, this study did not report its association with FPG or the relationship after adjusting for BMI or other covariates [21]. In addition, it did not report the association between B12 and BMI. These factors may have accounted for the differences, especially due to the inverse relationship observed between B12 and BMI in our study. This inverse relationship between B12 and BMI has been seen in other studies both during [10, 16, 17] and outside pregnancy [38].
Two recent Mendelian randomisation studies, primarily in white people, did not demonstrate a causal link between B12 and BMI [39, 40]. FUT2 polymorphisms can influence the composition of gut microbes [41] which in turn can cause both obesity and low B12. This was proposed as a potential explanation for the strong observational link but lack of Mendelian randomisation link between low B12 and high BMI [40]. Our in vitro studies showed that subcutaneous adipose tissue isolated at the time of delivery from pregnant women with low B12 levels had increased expression of adipogenic and lipogenic genes, as well as altered expression of 12 microRNAs (miRNAs) that regulate the peroxisome proliferator-activated receptor (PPAR)γ and insulin signalling pathways [38, 42]. These adipose tissue-derived miRNAs were also altered in the maternal circulation [42], which in turn can affect the beta cell function and/or hepatic handling of glucose levels [43, 44].
Our study provides clarification for conflicting data that have been reported recently on maternal folate levels and GDM risk. Our large sample size enabled us to demonstrate a U-shaped relationship between folate and FPG levels and may explain the opposite associations reported in different populations. High folate levels within the reference range may be beneficial for FPG but harmful for 2 h-PG, and folate levels above the reference range appear to be harmful for both. The relationship of higher folate and higher 2h-PG with GDM risk is similar to observations reported in Chinese [19,20,21] and Indian populations [15] but opposite to that in the American population [18], which only had estimated folate intake. The American study was conducted prior to mandatory folate fortification and hence was unlikely to have had the high levels of folate observed in our population. It has been shown that excess folic acid from supplements results in the presence of unmetabolised folic acid levels in serum in all age groups [45]. Unmetabolised folic acid slows the 1-C cycle, reduces the methylation potential and conversion of methionine, and increases intracellular accumulation of ATP [46]. This can lead to reduced insulin-mediated glucose uptake in muscle cells in vitro and in animal models, resulting in reduced insulin sensitivity and higher insulin resistance in the offspring [45, 46]. This may explain the opposite associations we observed for folate levels within the reference range with FPG and 2h-PG levels. In addition, a ‘low B12–high folate’ combination could have compromised the methylation potential further, which is known to be associated with adverse metabolic risk [47, 48].
tHcy had inverse associations with glucose levels and adjusting for BMI augmented its effect size on blood glucose level. Similar inverse associations between tHcy and glucose levels were also observed recently [15]. We found that higher BMI is associated with higher tHcy and glucose levels. In addition, it is known that high tHcy levels can be associated with low birthweight [49], and 5–7% of offspring of women with GDM may be small for gestational age [2, 3]. Further investigations are warranted to understand the complex relationships among tHcy, glucose levels and birthweight.
Strengths and limitations
The strengths of our study were that it was a large, early pregnancy, multi-ethnic, prospective cohort in a UK representative population, with more than 90% follow-up. We centrifuged our samples within 30 min, and measured folate up to 136 nmol/l and tHcy by the LCMS method. However, our study had the following limitations. First, we did not have glucose measurements in early pregnancy or 30 min or 1 h glucose and insulin measurements at OGTT to further explore the associations of B12/folate with beta cell function or insulin resistance. Lack of 1 h glucose levels may have underestimated the IADPSG-GDM rates. However, we believe this is unlikely to have influenced our conclusions, as the observed associations between these B vitamins and glucose levels were continuous. Second, we did not have oxidised folate (or sub-fractions of folate) measurements. While this is unlikely to have influenced our results, it may have helped to explore the relationship between unmetabolised folic acid and 2 h-PG. Third, the tHcy levels observed seem to be higher than in other studies. Our first trimester samples were non-fasted samples. Food is known to increase the tHcy levels [30]. In addition, our sensitive, LCMS-based assay could have contributed to these higher levels [50]. Fourth, as our study used NICE selective screening criteria, two-thirds of white women were screened because of obesity, and hence our findings may not be applicable to normal weight white women. Finally, although our cohort was relatively large, for our ethnic-specific analyses, it was still small, as shown by the wide CIs.
Implications, unanswered questions and future research
Our study demonstrated that B12 and folate levels may be independent, modifiable risk factors for hyperglycaemia in late pregnancy. B12 insufficiency is not uncommon in pregnancies across the world [51]. Our study confirmed this and showed that excess folate levels are also common, while folate deficiency was rare in early pregnancy, highlighting the need to avoid very high folate levels in early pregnancy. This warrants urgent review of the dose and duration of folic acid supplements and future research should focus on: (1) investigating the effect of optimising B12 and folate levels in early pregnancy and before conception on glucose levels and rate of GDM and/or its complications; (2) mechanistic studies investigating the effects of food- vs supplement-derived folate and unmetabolised folic acid levels on glucose levels and their potential epigenetic effects; and (3) studies designed to explore the complex relationships among tHcy, BMI, glucose and birthweight.
Data availability
Individual participant data that underlie the results reported in this article will be available from 9 to 36 months following the publication of this article. The data will be shared with researchers who provide a methodologically sound proposal that has been approved by an independent review committee to achieve the aims described in their proposal. Proposals should be directed to p.saravanan@warwick.ac.uk and Y.Weldeselassie@warwick.ac.uk to gain access. A data access agreement must be signed prior to access as per University of Warwick standard operating procedures.
Abbreviations
- aRR:
-
Adjusted RR
- BCAA:
-
Branched-chain amino acid
- 1-C:
-
Single carbon atoms
- FPG:
-
Fasting plasma glucose
- GDM:
-
Gestational diabetes mellitus
- 2 h-PG:
-
2 h post-load plasma glucose
- IADPSG:
-
International Association of Diabetes and Pregnancy Study Groups
- IADPSG-GDM:
-
GDM diagnosis based on IADPSG criteria
- LCMS:
-
Liquid chromatography mass spectrometry
- miRNA:
-
MicroRNA
- NICE:
-
National Institute for Health and Care Excellence
- NICE-GDM:
-
GDM diagnosis based on NICE criteria
- SES:
-
Socioeconomic status
- tHcy:
-
Total homocysteine
References
Saravanan P, Diabetes in Pregnancy Working Group, Maternal Medicine Clinical Study Group, Royal College of Obstetricians and Gynaecologists, UK (2020) Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol 8(9):793–800. https://doi.org/10.1016/S2213-8587(20)30161-3
Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS (2005) Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 352(24):2477–2486. https://doi.org/10.1056/NEJMoa042973
Landon MB, Spong CY, Thom E et al (2009) A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 361(14):1339–1348. https://doi.org/10.1056/NEJMoa0902430
Venkataraman H, Ram U, Craik S, Arungunasekaran A, Seshadri S, Saravanan P (2017) Increased fetal adiposity prior to diagnosis of gestational diabetes in South Asians: more evidence for the ‘thin-fat’ baby. Diabetologia 60(3):399–405. https://doi.org/10.1007/s00125-016-4166-2
Ram U, Seshadri S, Saravanan P (2017) Hyperglycaemia in pregnancy: time to ask the hard questions? Lancet Diabetes Endocrinol 5(8):578–579. https://doi.org/10.1016/S2213-8587(17)30175-4
Sovio U, Murphy HR, Smith GC (2016) Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care 39(6):982–987. https://doi.org/10.2337/dc16-0160
Saravanan P, Yajnik CS (2010) Role of maternal vitamin B12 on the metabolic health of the offspring: a contributor to the diabetes epidemic? B J Diab Vasc Disease 10:109–114. https://doi.org/10.1177/1474651409358015
Finer S, Saravanan P, Hitman G, Yajnik C (2014) The role of the one-carbon cycle in the developmental origins of type 2 diabetes and obesity. Diabet Med 31(3):263–272. https://doi.org/10.1111/dme.12390
Wang TJ, Larson MG, Vasan RS et al (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17(4):448–453. https://doi.org/10.1038/nm.2307
Boachie J, Adaikalakoteswari A, Samavat J, Saravanan P (2020) Low vitamin B12 and lipid metabolism: evidence from pre-clinical and clinical studies. Nutrients 12(7):1–19. 1925. https://doi.org/10.3390/nu12071925
Sinclair KD, Allegrucci C, Singh R et al (2007) DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A 104(49):19351–19356. https://doi.org/10.1073/pnas.0707258104
Clare CE, Brassington AH, Kwong WY, Sinclair KD (2019) One-carbon metabolism: linking nutritional biochemistry to epigenetic programming of long-term development. Annu Rev Anim Biosci 7:263–287. https://doi.org/10.1146/annurev-animal-020518-115206
Kouroglou E, Anagnostis P, Daponte A, Bargiota A (2019) Vitamin B12 insufficiency is associated with increased risk of gestational diabetes mellitus: a systematic review and meta-analysis. Endocrine 66(2):149–156. https://doi.org/10.1007/s12020-019-02053-1
Krishnaveni GV, Hill JC, Veena SR et al (2009) Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia 52(11):2350–2358. https://doi.org/10.1007/s00125-009-1499-0
Lai JS, Pang WW, Cai S et al (2018) High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin Nutr 37(3):940–947. https://doi.org/10.1016/j.clnu.2017.03.022
Knight BA, Shields BM, Brook A et al (2015) Lower circulating B12 is associated with higher obesity and insulin resistance during pregnancy in a non-diabetic white British population. PLoS One 10(8):e0135268. https://doi.org/10.1371/journal.pone.0135268
Sukumar N, Venkataraman H, Wilson S et al (2016) Vitamin B12 status among pregnant women in the UK and its association with obesity and gestational diabetes. Nutrients 8(12):1–10. 768. https://doi.org/10.3390/nu8120768
Li M, Li S, Chavarro JE et al (2019) Prepregnancy habitual intakes of total, supplemental, and food folate and risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care 42(6):1034–1041. https://doi.org/10.2337/dc18-2198
Li Q, Zhang Y, Huang L et al (2019) High-dose folic acid supplement use from prepregnancy through midpregnancy is associated with increased risk of gestational diabetes mellitus: a prospective cohort study. Diabetes Care 42(7):e113–e115. https://doi.org/10.2337/dc18-2572
Zhu B, Ge X, Huang K et al (2016) Folic acid supplement intake in early pregnancy increases risk of gestational diabetes mellitus: evidence from a prospective cohort study. Diabetes Care 39(3):e36–e37. https://doi.org/10.2337/dc15-2389
Chen X, Zhang Y, Chen H et al (2020) Association of maternal folate and vitamin B12 in early pregnancy with gestational diabetes mellitus: a prospective cohort study. Diabetes Care 4(1):217–223. https://doi.org/10.2337/dc20-1607
Setola E, Monti LD, Galluccio E et al (2004) Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patients with metabolic syndrome: relationship between homocysteine levels and hyperinsulinemia. Eur J Endocrinol 151(4):483–489. https://doi.org/10.1530/eje.0.1510483
Guven MA, Kilinc M, Batukan C, Ekerbicer HC, Aksu T (2006) Elevated second trimester serum homocysteine levels in women with gestational diabetes mellitus. Arch Gynecol Obstet 274(6):333–337. https://doi.org/10.1007/s00404-006-0191-6
Mascarenhas M, Habeebullah S, Sridhar MG (2014) Revisiting the role of first trimester homocysteine as an index of maternal and fetal outcome. J Pregnancy 2014:123024. https://doi.org/10.1155/2014/123024
Seghieri G, Breschi MC, Anichini R et al (2003) Serum homocysteine levels are increased in women with gestational diabetes mellitus. Metab Clin Exp 52(6):720–723. https://doi.org/10.1016/S0026-0495(03)00032-5
Tarim E, Bagis T, Kilicdag E et al (2004) Elevated plasma homocysteine levels in gestational diabetes mellitus. Acta Obstet Gynecol Scand 83(6):543–547. https://doi.org/10.1111/j.0001-6349.2004.00540.x
NICE (2015) Diabetes in pregnancy: management from preconception to the postnatal period NICE guidelines [NG3]. Available from https://www.nice.org.uk/guidance/ng3. Accessed 22 May 2021
Adaikalakoteswari A, Webster C, Goljan I, Saravanan P (2016) Simultaneous detection of five one-carbon metabolites in plasma using stable isotope dilution liquid chromatography tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 1012-1013:186–192. https://doi.org/10.1016/j.jchromb.2016.01.026
United Hospital Birmingham NHS Foundation Trust (2016) Homocysteine - General Information. Available from https://heftpathology.com/item/homocysteine-homocystine-hcys.html. Accessed 22 May 2021
Refsum H, Smith AD, Ueland PM et al (2004) Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 50(1):3–32. https://doi.org/10.1373/clinchem.2003.021634
Metzger BE, Gabbe SG, Persson B et al (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33(3):676–682. https://doi.org/10.2337/dc09-1848
Rogne T, Tielemans MJ, Chong MF et al (2017) Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birth weight: a systematic review and meta-analysis of individual participant data. Am J Epidemiol 185(3):212–223. https://doi.org/10.1093/aje/kww212
Stabler SP (2013) Clinical practice. Vitamin B12 deficiency. N Engl J Med 368(2):149–160. https://doi.org/10.1056/NEJMcp1113996
Sukumar N, Adaikalakoteswari A, Venkataraman H, Maheswaran H, Saravanan P (2016) Vitamin B12 status in women of childbearing age in the UK and its relationship with national nutrient intake guidelines: results from two National Diet and Nutrition Surveys. BMJ Open 6(8):e011247. https://doi.org/10.1136/bmjopen-2016-011247
Devalia V, Hamilton MS, Molloy AM, British Committee for Standards in Haematology (2014) Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol 166(4):496–513. https://doi.org/10.1111/bjh.12959
French SA, Tangney CC, Crane MM, Wang Y, Appelhans BM (2019) Nutrition quality of food purchases varies by household income: the SHoPPER study. BMC Public Health 19(1):231. https://doi.org/10.1186/s12889-019-6546-2
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Adaikalakoteswari A, Finer S, Voyias PD et al (2015) Vitamin B12 insufficiency induces cholesterol biosynthesis by limiting s-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clin Epigenetics 7(1):14. https://doi.org/10.1186/s13148-015-0046-8
Moen GH, Qvigstad E, Birkeland KI, Evans DM, Sommer C (2018) Are serum concentrations of vitamin B-12 causally related to cardiometabolic risk factors and disease? A Mendelian randomization study. Am J Clin Nutr 108(2):398–404. https://doi.org/10.1093/ajcn/nqy101
Allin KH, Friedrich N, Pietzner M et al (2017) Genetic determinants of serum vitamin B12 and their relation to body mass index. Eur J Epidemiol 32(2):125–134. https://doi.org/10.1007/s10654-016-0215-x
Wacklin P, Tuimala J, Nikkila J et al (2014) Faecal microbiota composition in adults is associated with the FUT2 gene determining the secretor status. PLoS One 9(4):e94863. https://doi.org/10.1371/journal.pone.0094863
Adaikalakoteswari A, Vatish M, Alam MT, Ott S, Kumar S, Saravanan P (2017) Low vitamin B12 in pregnancy is associated with adipose-derived circulating miRs targeting PPARgamma and insulin resistance. J Clin Endocrinol Metab 102(11):4200–4209. https://doi.org/10.1210/jc.2017-01155
Trajkovski M, Hausser J, Soutschek J et al (2011) MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 474(7353):649–653. https://doi.org/10.1038/nature10112
Ying W, Riopel M, Bandyopadhyay G et al (2017) Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 171(2):372–384 e312. https://doi.org/10.1016/j.cell.2017.08.035
Maruvada P, Stover PJ, Mason JB et al (2020) Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr 112(5):1390–1403. https://doi.org/10.1093/ajcn/nqaa259
Bayer AL, Fraker CA (2017) The folate cycle as a cause of natural killer cell dysfunction and viral etiology in type 1 diabetes. Front Endocrinol (Lausanne) 8:315. https://doi.org/10.3389/fendo.2017.00315
Samblas M, Milagro FI, Martinez A (2019) DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics 14(5):421–444. https://doi.org/10.1080/15592294.2019.1595297
Wu P, Farrell WE, Haworth KE et al (2018) Maternal genome-wide DNA methylation profiling in gestational diabetes shows distinctive disease-associated changes relative to matched healthy pregnancies. Epigenetics 13(2):122–128. https://doi.org/10.1080/15592294.2016.1166321
Hogeveen M, Blom HJ, den Heijer M (2012) Maternal homocysteine and small-for-gestational-age offspring: systematic review and meta-analysis. Am J Clin Nutr 95(1):130–136. https://doi.org/10.3945/ajcn.111.016212
Pfeiffer CM, Huff DL, Smith SJ, Miller DT, Gunter EW (1999) Comparison of plasma total homocysteine measurements in 14 laboratories: an international study. Clin Chem 45(8 Pt 1):1261–1268
Sukumar N, Rafnsson SB, Kandala NB, Bhopal R, Yajnik CS, Saravanan P (2016) Prevalence of vitamin B-12 insufficiency during pregnancy and its effect on offspring birth weight: a systematic review and meta-analysis. Am J Clin Nutr 103(5):1232–1251. https://doi.org/10.3945/ajcn.115.123083
Acknowledgements
We acknowledge the following team members, collaborators and other staff who have contributed to this study. George Eliot Hospital NHS Trust: G. Pounder, J. Plester, S. Selvamoni, J. Farmer, J. Duffy, K. Shorthose, G. Sutton, C. Wood, N. Andersen, C. Webster (Heartlands Hospital pathology department, Birmingham); University Hospital Coventry and Warwickshire: S. Keay (principal investigator [PI]), S. Quenby (PI), N. Flint, N. Morris, H. Usher; Warwick Hospital NHS Trust: O. Sorinola (PI), Z. D’Souza, A. Guy, K. Jukes; The Royal Wolverhampton NHS Trust: R. Raghavan (PI), J. Icke, K. Cheshire, L. Devison, K. Vassell, C. Busby, L. Bibb, P. Mhembere; Worcestershire Royal Hospital: L. Thirumalaikumar (PI), D. Kelly, V. Cashmore, S. Raine, S. Tohill, C. Alton, K. McDonald, K. Townsend; The Leeds Teaching Hospitals NHS Trust: E. Scott (PI), S. Nettleton, M. Home, R. Hudson, S. Ives, A. Proctor, L. Lord, J. Towning, W. Andrusjak, A. Scott, D. Endersby, K. Robinson, J. Parker; The Shrewsbury and Telford Hospital NHS Trust: S. Hodgett (PI), M. Beekes, J. Jones, H. Millward, F. Hurford, R. Wilcox; Queens Medical Centre, Nottingham: D. Kapoor (PI), Y. Davis, C. Wilson, C. Hussain, L. Common; Nottingham City Hospital: D. Kapoor (PI), V. May, C. Smith, G. Kirkwood, J. Longmate; York Teaching Hospital NHS Foundation Trust: P. Jennings (PI), H. Hancock, S. Roche, D. Thompson. We also acknowledge S. Sampathkumar and S. Chockalingam for their help with formatting of the tables.
Authors’ relationships and activities
All authors have completed the Unified Competing Interest Form and declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
The study was funded by the Medical Research Council (MRC), UK (MR/J000094/1). The study funder was not involved in the design of the study; the collection, analysis and interpretation of the data; writing the report; and did not impose any restrictions regarding the publication of the report.
Author information
Authors and Affiliations
Contributions
PS conceived the idea, designed the study and developed this further with CHDF and CSY and obtained funding. PS oversaw the recruitment of centres and overall conduct of the study with the help of N. Sukumar and AG. N. Sukumar, HV and CB contributed to day-to-day running of the study alongside their PhD sub-studies at various stages. AG was the research coordinator and IG was the biobank coordinator and completed the B12 and folate analyses. AA developed, validated and completed the LCMS analyses. N. Stallard designed the statistical aspect of the study. YG-W conducted all aspects of the analysis with the support of N. Stallard. All authors contributed to the interpretation of results and writing of the manuscript and approved the final version. PS is the guarantor of this work, and accepts full responsibility for the work; had access to the data, integrity and accuracy of the data analysis; and controlled the decision to publish.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 544 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saravanan, P., Sukumar, N., Adaikalakoteswari, A. et al. Association of maternal vitamin B12 and folate levels in early pregnancy with gestational diabetes: a prospective UK cohort study (PRiDE study). Diabetologia 64, 2170–2182 (2021). https://doi.org/10.1007/s00125-021-05510-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05510-7