Abstract
Aims/hypothesis
Elevated circulating adipocyte fatty acid-binding protein (AFABP) levels have been found to correlate with diabetic nephropathy staging in cross-sectional studies. However, it remains unclear whether these higher serum levels reflect a role of AFABP in the development of diabetic kidney disease (DKD), or simply result from its impaired renal clearance in DKD. Here we investigated prospectively the prognostic importance of serum AFABP level in the development of adverse renal outcomes in a large clinic-based cohort of participants with type 2 diabetes.
Methods
Baseline serum AFABP levels were measured in 5454 Chinese participants from the Hong Kong West Diabetes Registry. The association between circulating AFABP levels and incident adverse renal outcomes—defined as a composite endpoint of a sustained 40% decline in eGFR, end-stage renal disease requiring renal replacement therapy or kidney transplantation, or renal deaths—was evaluated using multivariable Cox regression analysis.
Results
Over a median follow-up of 5 years, 754 of the 5454 participants developed incident adverse renal outcomes. Elevated circulating AFABP levels were independently associated with incident adverse renal outcomes (HR 1.43, 95% CI 1.31, 1.57, p < 0.001) after adjustments for conventional risk factors for DKD progression. Importantly, the prognostic role of serum AFABP was independent of the baseline albuminuria status or eGFR levels of the study participants.
Conclusions/interpretation
Circulating AFABP levels were predictive of incident adverse renal outcomes, even in participants with relatively well-preserved kidney function at baseline, suggesting its potential to be a useful marker for early risk stratification in DKD.
Similar content being viewed by others

Introduction
Diabetic kidney disease (DKD) is a major chronic diabetic complication and it is one of the leading causes of end-stage renal disease (ESRD) and renal deaths worldwide. A recent study reported that more than a quarter of US adults with diabetes have DKD [1]. Similarly, in China, approximately 21.3% of individuals with diabetes are affected and the estimated number of individuals with DKD is close to 24.3 million [2]. DKD, be it albuminuria, impaired eGFR or both, is associated not only with adverse cardiovascular and renal outcomes [3], but also with increased all-cause mortality in individuals with type 2 diabetes [4]. Although microalbuminuria used to be the most studied marker of DKD progression [5, 6], the classical paradigm of microalbuminuria always preceding macroalbuminuria and eGFR decline has been challenged over the past decade. In a 15-year follow-up study of the United Kingdom Prospective Diabetes Study, of the 1534 participants who had albuminuria, 12% were documented to have renal impairment (defined as an eGFR <60 ml min−1 [1.73 m]−2 or doubling of the serum creatinine levels) before the detection of albuminuria. Furthermore, 51% of the 1132 participants who had renal impairment did not have albuminuria. These observations have highlighted the apparent discordance between the development of impaired eGFR and albuminuria as well as the limitations of using microalbuminuria alone to optimally identify individuals with type 2 diabetes who are at higher risk of renal impairment [7]. Therefore, well-validated novel markers for adverse renal outcomes are clearly needed to provide good prognostic information for the vast majority of individuals with type 2 diabetes [8].
Adipocyte fatty acid-binding protein (AFABP) is a lipid chaperone that mediates intracellular fatty acid trafficking [9]. It is also highly expressed in macrophages [10] and it modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinase (JNK) and activator protein-1 [11]. In humans, we and others have previously demonstrated serum AFABP to be higher in obesity [12], and elevated serum AFABP levels were independently associated with the development of the metabolic syndrome [13], type 2 diabetes [14, 15], incident cardiovascular events and heart failure in community-based cohorts [16, 17], as well as all-cause mortality, cardiovascular- and infection-related deaths in type 2 diabetes [18]. In the context of DKD, a high serum AFABP level was independently associated with diabetic nephropathy staging based on previous cross-sectional studies [19,20,21]. However, in view of the cross-sectional design of these studies and the fact that filtration through the kidneys is an important route of AFABP clearance, which could also account for the elevated serum levels in DKD [22], the relationship between circulating AFABP levels and DKD remains to be elucidated. Therefore, we performed this prospective study to examine the prognostic importance of serum AFABP level in the development of adverse renal outcomes in individuals with type 2 diabetes using a large clinic-based cohort of Chinese participants.
Methods
Study participants
All participants were recruited from the Hong Kong West Diabetes Registry, which consisted of individuals who had type 2 diabetes and had been followed up regularly at the medical specialist clinics of the Hong Kong West Cluster since 2008. During enrolment to the registry, all Chinese individuals were invited to participate in a prospective cohort study that aimed to identify the associations between risk factors, including genetic and serum biomarkers, and the development of diabetic complications; this involved regular assessment for chronic diabetic complications every 12 to 18 months, in addition to their usual follow-up for diabetes every 4 to 6 months according to a management protocol. During each assessment, the participants were assessed clinically and had laboratory investigations to determine their control of diabetes, its related cardiovascular risk factors and the presence of chronic diabetic complications, which included the development of albuminuria and decline in renal function. The study protocol was approved by the institutional review board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. Written informed consent was obtained from all recruited participants prior to any study-related procedures. In the current study that evaluated the role of circulating AFABP levels and incident adverse renal outcomes, only individuals who had follow-up for more than 1 year were included. Individuals who were non-Chinese, those who were on renal replacement therapy or those who had received a kidney transplant at baseline were excluded.
Clinical and biochemical assessments
The participants attended each assessment after an overnight fast for at least 8 h. At the baseline assessment, demographic data were obtained, including age, sex, smoking status and alcohol consumption. Detailed medical, family and medication histories were assessed using a standardised questionnaire. Anthropometric parameters were measured, including body weight, height, BMI, waist circumference (WC) and BP. Fasting blood was drawn for plasma glucose, lipids and HbA1c levels and stored in aliquots at −70°C for assays of biomarkers of diabetic complications. Serum creatinine level was measured. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation and it was expressed as GFR = 141 × min(SCr/κ,1)α × max (SCr/κ,1)−1.209 × 0.993age (×1.018 for women), where SCr is serum creatinine (expressed in mg/dl; if converting from μmol/l to mg/dl, divide by 88.4), κ is 0.7 for women and 0.9 for men, α is −0.329 for women and −0.411 for men, min indicates the minimum of SCr/κ or 1, and max indicates the maximum of SCr/κ or 1 [23]. Albuminuria status was assessed using at least two random urine samples on two separate occasions within 6 months and was categorised accordingly (urine albumin/creatinine ratio <3 mg/mmol [A1], 3–30 mg/mmol [A2] and >30 mg/mmol [A3]) [24]. Serum high-sensitivity C-reactive protein (hsCRP) level was measured with a high-sensitivity, particle-enhanced immune-turbidimetric assay (Roche Diagnostics, Mannheim, Germany). Serum AFABP was measured with a monoclonal-antibody-based ELISA (Antibody and Immunoassay Services, University of Hong Kong) that had also been used in other regional and international studies [25, 26]. The intra-assay and inter-assay precision coefficients of variation of the AFABP ELISA were <4.1% and <4.5%, respectively, and the lowest detection limit was 0.39 ng/ml.
Definitions of clinical variables and outcomes
Dyslipidaemia was defined as fasting triacylglycerol ≥1.69 mmol/l, HDL-cholesterol (HDL-C) <1.04 mmol/l in men and <1.29 mmol/l in women, LDL-cholesterol (LDL-C) ≥2.6 mmol/l or the use of lipid-lowering agents. Hypertension was defined as BP ≥140/90 mmHg or the use of anti-hypertensive medications.
Adverse renal outcomes, the primary outcome of interest in this study, were defined [27] as the development of a composite endpoint consisting of a 40% decline in eGFR that was sustained for at least two consecutive measurements, initiation of renal replacement therapy, the need for kidney transplantation, or death from renal causes. All outcomes were recorded and verified from the Hospital Authority database or the private practitioners of the participants as of 30 June 2017. Deaths from renal causes were based on coding from the 9th edition of the International Classification of Diseases (ICD-9) (585.6) (www.icd9data.com/2007/Volume1).
Statistical analysis
All data were analysed with IBM SPSS Statistics 24.0.0 (http://www.IBM.com/SPSS) and R version 3.2.3 (http://www.r-project.org). Data that were not normally distributed as determined using the Kolmogorov–Smirnov test, which included serum AFABP, triacylglycerol and hsCRP levels, were log2 transformed before analysis. The values were reported as means ± SD, medians with interquartile range with skewed data, or percentages. Multivariable Cox regression analysis was conducted to evaluate the associations between baseline serum AFABP levels and incident adverse renal outcomes. The variables included in the Cox regression models were those that were either statistically significant in the univariate analysis or biologically relevant [28]. Interactions were tested using multivariable Cox regression. Effect modification among sex×AFABP or sex×WC was tested using a two-way interaction and the interaction was observed only in the latter. The HR for circulating AFABP level referred to the risk of developing adverse renal outcomes per unit difference in the log-transformed, or a doubling of serum AFABP level measured in ng/ml. The adjusted cumulative incidence curves were derived from a multivariable Cox regression model using the mean of each covariate as a constant. The predictive performance of the various models was assessed using C index (the AUC of the ROC curve, also known as the C statistic), category-free net reclassification index (NRI) and integrated discrimination improvement (IDI). In all statistical tests, two-sided p values <0.05 were considered significant.
Results
Circulating AFABP levels were significantly higher in participants with incident adverse renal outcomes
A total of 5454 participants with type 2 diabetes were included in our study, after excluding 50, 15 and 150 participants who were on renal replacement therapy, who underwent renal transplantation and who were either lost to follow-up or had less than 1 year of follow-up, respectively. Over a median follow-up of 5 years, 754 (13.8%) participants developed adverse renal outcomes: 703 participants had a 40% decline in eGFR; 40 participants presented with ESRD and required renal replacement therapy or kidney transplantation; and 11 participants died from renal causes. The participants who had incident adverse renal outcomes were significantly older, and they had longer duration of diabetes, higher WC and BP, worse glycaemic control, higher LDL-C and triacylglycerol levels, and higher prevalence of cardiovascular disease at baseline than those who did not. In addition, those who developed adverse renal outcomes had a significantly lower eGFR and more severe albuminuria at baseline, despite a higher proportion of participants who were taking an ACE inhibitor (ACEI) or angiotensin II receptor blocker (ARB). Both serum hsCRP and AFABP levels at baseline were significantly higher in participants who developed adverse renal outcomes than those who did not (see electronic supplementary material [ESM] Table 1). Moreover, at baseline, higher serum AFABP quartiles were significantly associated with older age; longer duration of diabetes; lower eGFR levels; more severe albuminuria; higher WC, systolic BP, serum HbA1c, triacylglycerol and hsCRP levels; higher prevalence of hypertension and dyslipidaemia; history of cardiovascular disease; and history of using thiazolidinedione, insulin, ACEIs/ARBs, statin and aspirin (all p < 0.001) (Table 1).
Circulating AFABP levels were independently associated with incident adverse renal outcomes
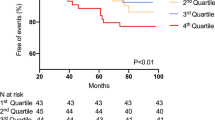
In the multivariable Cox regression analysis (Table 2), baseline serum AFABP level was independently associated with the development of adverse renal outcomes after 5 years (HR 1.43, 95% CI 1.31, 1.57; p < 0.001), along with sex (p = 0.008), age (p < 0.001), interaction term for sex and WC (p = 0.027), systolic BP (p < 0.001), eGFR (p < 0.001), albuminuria status (p < 0.001), use of insulin (p = 0.042) and serum hsCRP levels (p = 0.01) at baseline, in a full model that also included smoking status, duration of diabetes, WC, hypertension, LDL-C level, triacylglycerol level, history of cardiovascular disease, HbA1c level and the use of ACEIs or ARBs, statin and aspirin at baseline. Serum AFABP level remained independently associated with adverse renal outcomes even when eGFR was adjusted as a continuous variable (HR 1.37, 95% CI 1.25, 1.51; p < 0.001) or in a reduced model that used Akaike’s information criterion (HR 1.42, 95% CI 1.30, 1.56; p < 0.001). Moreover, increasing serum AFABP quartiles were significantly associated with a higher adjusted cumulative incidence of adverse renal outcomes (p < 0.001) (Fig. 1). Multivariable spline showed a non-linear increasing trend in the adjusted HRs of incident adverse renal outcomes, and the risks appeared to increase when serum AFABP rose beyond the second quartile (ESM Fig. 1).
Adjusted cumulative incidence of adverse renal outcomes with increasing quartiles of circulating AFABP levels. Adjusted p < 0.001. Model was adjusted for sex, age, smoking, duration of diabetes, WC, interaction term for sex and WC, systolic BP, history of cardiovascular disease, HbA1c, triacylglycerol, LDL-C, eGFR, albuminuria categories, use of ACEIs/ARBs, use of insulin, use of statin, use of aspirin, and serum hsCRP at baseline (as in Model 3, see Table 2)
The prognostic role of circulating AFABP levels was independent of sex, baseline obesity, eGFR and albuminuria status
To confirm the robustness of the observed associations between baseline serum AFABP levels and incident adverse renal outcomes, subgroup analyses were performed to examine the prognostic properties of circulating AFABP levels in the participants stratified by sex, their baseline eGFR categories, obesity and albuminuria status (Table 3). Although we observed higher baseline serum AFABP levels in women than in men, as well as worsening eGFR (ESM Table 2), the prognostic properties of serum AFABP levels for the development of adverse renal outcomes remained significant irrespective of sex (HR 1.50, 95% CI 1.32, 1.70; p < 0.001 in men and HR 1.32, 95% CI 1.15, 1.52; p < 0.001 in women), obesity status (HR 1.45, 95% CI 1.23, 1.70; p < 0.001 for BMI ≥27.5 kg/m2 and HR 1.44, 95% CI 1.28, 1.62; p < 0.001 for BMI <27.5 kg/m2), albuminuria status (HR 1.32, 95% CI 1.07, 1.63; p = 0.009 for A1; HR 1.43, 95% CI 1.19, 1.71; p < 0.001 for A2; and HR 1.43, 95% CI 1.26, 1.63; p < 0.001 for A3) and eGFR categories (HR 1.37, 95% CI 1.19, 1.56; p < 0.001 for eGFR ≥60 ml min−1 [1.73 m]−2 and HR 1.29, 95% CI 1.12, 1.50; p < 0.001 for eGFR <60 ml min−1 [1.73 m]−2) at baseline. Furthermore, in a subgroup of 2537 study participants with relatively well-preserved kidney function at baseline, as defined by eGFR ≥60 ml min−1 [1.73 m]−2 and normoalbuminuria, circulating AFABP levels remained independently associated with development of adverse renal outcomes (HR 1.41, 95% CI 1.12, 1.78, p = 0.004).
Circulating AFABP levels provided a significant improvement in prognostic performance for incident adverse renal outcomes, particularly in participants with less severe albuminuria and even normoalbuminuria with preserved kidney function
Since the prognostic properties of circulating AFABP levels for incident adverse renal outcomes were independent of baseline albuminuria and eGFR levels, we further determined the C indices, NRI and IDI to examine the clinical usefulness of employing circulating AFABP levels in participants with different levels of albuminuria at baseline. In all participants, circulating AFABP levels provided significant improvement in the prognostic performance for incident adverse renal outcomes, as shown in the increments in C indices (from 0.816 to 0.823, p = 0.0008), NRI (19%, 95% CI 13.1, 23.3; p < 0.001) and IDI (1.6%, 95% CI 0.9, 2.4; p < 0.001) after the addition of circulating AFABP levels to the model (Table 4). Remarkably, these significant improvements in C indices, NRI and IDI were similar across participants with all albuminuria categories from A1 to A3 at baseline.
Discussion
In this study, we have provided the novel observation that serum AFABP level is independently associated with the development of adverse renal outcomes in type 2 diabetes over a median follow-up of 5 years, and have demonstrated the potential of employing circulating AFABP levels as a useful prognostic marker for disease progression across different stages of DKD.
Both albuminuria and impaired eGFR are robust independent predictors of ESRD and renal death [3, 29]. Nonetheless, we found that the association between circulating AFABP levels and adverse renal outcomes remained significant even after adjusting for the baseline eGFR levels and albuminuria status of the study participants. This was confirmed by further analyses of participants stratified by their baseline albuminuria status or eGFR categories, and of the subgroup of participants with relatively well-preserved kidney function at baseline. Moreover, the improvements in NRI and IDI as well as the modest but significant increments in C indices were both observed in participants with different levels of albuminuria, including normoalbuminuria. The implications of these findings were twofold. First, a good prognostic marker should enable early risk stratification and, as a result, timely intervention could be provided to prevent DKD progression. In this regard, we have shown that circulating AFABP levels were predictive of adverse renal outcomes over and above known predictors of ESRD and renal deaths, even in participants with eGFR ≥60 ml min−1 [1.73 m]−2 and normoalbuminuria. This is particularly relevant as emerging evidence from both large-scale observational and epidemiological studies have suggested changes in the clinical manifestations of DKD, with an increasing prevalence of impaired eGFR without accompanying albuminuria being observed [1, 7]. Second, although circulating AFABP levels were known to be higher in individuals who were on chronic haemodialysis and in those with chronic kidney disease and even acute renal dysfunction [22, 30], it was unlikely that the increased serum AFABP levels in DKD simply reflect an impaired renal clearance because their prognostic properties remained significant even after adjusting for the baseline kidney function of the study participants. Furthermore, despite the sexual dimorphism commonly found in serum AFABP levels [12], we did not observe a significant difference between the sexes in their associations with adverse renal outcomes in the subgroup analysis.
Our findings also highlighted the role of inflammation, among the multiple factors implicated, in the pathogenesis of DKD [31]. A recent study based on the Action to Control Cardiovascular Risk in Type 2 Diabetes (ACCORD) and Veterans Affairs Nephropathy in Diabetes (VA-NEPHRON-D) cohorts has also shown that the biomarkers of inflammation including tumour necrosis factor receptor (TNFR)-1, TNFR-2, and kidney injury molecule-1 (KIM-1) were independently associated with a higher risk of eGFR decline in DKD [8]. Although AFABP was higher in obesity [12], in the current study, neither BMI nor WC alone was independently associated with adverse renal outcomes. Since AFABP is also expressed in macrophages [10] and macrophage accumulation was observed in the glomeruli and interstitium of individuals with diabetic nephropathy [32], we speculate that AFABP might be related to the pathogenesis of DKD through amplification of the expression of proinflammatory cytokines early in the course of diabetic nephropathy [33, 34]. Indeed, previous in vitro studies performed by our group had shown that AFABP could form a positive feedback loop with JNK, synergistically perpetuating macrophage infiltration and adipose tissue inflammation, leading to a heightened expression of proinflammatory cytokines including monocyte chemoattractant protein-1 (MCP-1), TNF-α and IL-6 [11]. The effect of AFABP administration on enhancing the expression of these proinflammatory cytokines was also observed in studies in vivo [35]. Moreover, our findings showed that circulating AFABP levels remained independently associated with adverse renal outcomes even after adjusting for baseline serum hsCRP level, a well-established marker of systemic inflammation; this result suggested that AFABP might have an impact on DKD through pathways independent or upstream of C-reactive protein, the hepatic synthesis of which is induced by proinflammatory cytokines such as IL6 and TNF-α in the liver [36]. Recently, AFABP expression was also observed in the glomeruli of individuals with DKD [37, 38]. Importantly, the increased expression of AFABP in glomerular mesangial cells was accompanied by the increased expression of endoplasmic reticulum (ER) stress markers 78 kDa glucose-regulated protein (GRP78) and caspase-12, and a decreased expression of B cell CLL/lymphoma 2 (Bcl2). In vitro studies by the same group also showed that the inhibition of AFABP, either pharmacologically or genetically, attenuated non-esterified fatty acid- or high-glucose-induced ER stress as well as ER stress-mediated apoptosis. Although the extent to which this increased glomerular AFABP expression relates to the elevated circulating levels observed in our study requires further characterisation, findings from these studies suggested that AFABP might also mediate ER stress-induced glomerular cell apoptosis in DKD [38].
The present study had several limitations. First, renal biopsy was not performed in the majority of participants in our study cohort; hence, the histological diagnosis of DKD could not be confirmed. Second, the majority of outcome events were attributed to a 40% decline in eGFR, as our follow-up duration was relatively short for the observation of ESRD and renal deaths, therefore limiting further analyses on specific adverse renal outcomes. Third, the inclusion of only Chinese participants could limit the ability to generalise our findings. Moreover, comparison between serum AFABP and other novel renal biomarkers such as TNFR-1, TNFR-2 or KIM-1 was not possible because these biomarkers were not measured in our study. Last but not least, because of the cohort design, 24 h urine collection for the documentation of renal clearance of AFABP was not performed; hence, residual confounding might still be possible. Nonetheless, the strengths of our study include the large sample size of exclusively participants with type 2 diabetes, the evaluation of albuminuria status with at least two randomly collected urine samples to minimise misclassification, and the use of a clinically relevant composite endpoint of adverse renal outcomes, the occurrence of which had been shown to decrease with the use of sodium–glucose cotransporter 2 inhibitors [27, 39].
To conclude, we have demonstrated the prognostic importance of circulating AFABP levels in the development of adverse renal outcomes in type 2 diabetes, which was especially useful among those with relatively well-preserved kidney function and normoalbuminuria at baseline. However, further studies are required to validate our findings. Over the years, DKD has remained a devastating complication of type 2 diabetes, and until recently, therapeutics have been limited to ACEIs or ARBs only. Furthermore, data from a US study has shown that, despite the increased use of renin–angiotensin system blockers in recent decades, there has been no significant reduction in mortality due to renal causes in individuals with diabetes, whose risk of dying from renal causes remains threefold higher than those without diabetes [40]. Therefore, the current study does not only present a novel marker potentially useful for early risk stratification for DKD progression, but also prompts further research to unravel the complex pathogenesis of DKD, and to develop novel therapeutics that improve the overall standards of care in type 2 diabetes.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACEI:
-
ACE inhibitor
- AFABP:
-
Adipocyte fatty acid-binding protein
- ARB:
-
Angiotensin II receptor blocker
- DKD:
-
Diabetic kidney disease
- ER:
-
Endoplasmic reticulum
- ESRD:
-
End-stage renal disease
- HDL-C:
-
HDL-cholesterol
- hsCRP:
-
High-sensitivity C-reactive protein
- IDI:
-
Integrated discrimination improvement
- JNK:
-
c-Jun NH2-terminal kinase
- KIM-1:
-
Kidney injury molecule-1
- LDL-C:
-
LDL-cholesterol
- NRI:
-
Net reclassification index
- TNFR:
-
Tumour necrosis factor receptor
- WC:
-
Waist circumference
References
Afkarian M, Zelnick LR, Hall YN et al (2016) Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 316:602–610. https://doi.org/10.1001/jama.2016.10924
Zhang L, Long J, Jiang W et al (2016) Trends in chronic kidney disease in China. N Engl J Med 375:905–906. https://doi.org/10.1056/NEJMc1602469
Ninomiya T, Perkovic V, de Galan BE et al (2009) Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20:1813–1821. https://doi.org/10.1681/ASN.2008121270
Afkarian M, Sachs MC, Kestenbaum B et al (2013) Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 24:302–308. https://doi.org/10.1681/ASN.2012070718
Mogensen CE (1984) Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med 310:356–360. https://doi.org/10.1056/NEJM198402093100605
Berrut G, Bouhanick B, Fabbri P et al (1997) Microalbuminuria as a predictor of a drop in glomerular filtration rate in subjects with non-insulin-dependent diabetes mellitus and hypertension. Clin Nephrol 48:92–97
Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, UKPDS Study Group (2006) Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55:1832–1839. https://doi.org/10.2337/db05-1620
Coca SG, Nadkarni GN, Huang Y et al (2017) Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 28:2786–2793. https://doi.org/10.1681/ASN.2016101101
Boord JB, Fazio S, Linton MF (2002) Cytoplasmic fatty acid-binding proteins: emerging roles in metabolism and atherosclerosis. Curr Opin Lipidol 13:141–147. https://doi.org/10.1097/00041433-200204000-00005
Makowski L, Boord JB, Maeda K et al (2001) Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med 7:699–705. https://doi.org/10.1038/89076
Hui X, Li H, Zhou Z et al (2010) Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein-1. J Biol Chem 285:10273–10280. https://doi.org/10.1074/jbc.M109.097907
Xu A, Wang Y, Xu JY et al (2006) Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem 52:405–413. https://doi.org/10.1373/clinchem.2005.062463
Xu A, Tso AW, Cheung BM et al (2007) Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation 115:1537–1543. https://doi.org/10.1161/CIRCULATIONAHA.106.647503
Tso AW, Xu A, Sham PC et al (2007) Serum adipocyte fatty acid binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care 30:2667–2672. https://doi.org/10.2337/dc07-0413
Nowak C, Sundstrom J, Gustafsson S et al (2016) Protein biomarkers for insulin resistance and type 2 diabetes risk in two large community cohorts. Diabetes 65:276–284. https://doi.org/10.2337/db15-0881
Chow WS, Tso AW, Xu A et al (2013) Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: a 12-year prospective study. J Am Heart Assoc 2:e004176
Djousse L, Bartz TM, Ix JH et al (2013) Fatty acid-binding protein 4 and incident heart failure: the Cardiovascular Health Study. Eur J Heart Fail 15:394–399. https://doi.org/10.1093/eurjhf/hfs196
Lee CH, Cheung CYY, Woo YC et al (2018) Circulating adipocyte fatty acid-binding protein concentrations predict multiple mortality outcomes among men and women with diabetes. Clin Chem. https://doi.org/10.1373/clinchem.2018.289157
Cabre A, Lazaro I, Girona J et al (2008) Plasma fatty acid-binding protein 4 increases with renal dysfunction in type 2 diabetic patients without microalbuminuria. Clin Chem 54:181–187. https://doi.org/10.1373/clinchem.2007.094672
Yeung DC, Xu A, Tso AW et al (2009) Circulating levels of adipocyte and epidermal fatty acid-binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. Diabetes Care 32:132–134. https://doi.org/10.2337/dc08-1333
Toruner F, Altinova AE, Akturk M et al (2011) The relationship between adipocyte fatty acid binding protein-4, retinol binding protein-4 levels and early diabetic nephropathy in patients with type 2 diabetes. Diabetes Res Clin Pract 91:203–207. https://doi.org/10.1016/j.diabres.2010.11.011
Sommer G, Ziegelmeier M, Bachmann A et al (2008) Serum levels of adipocyte fatty acid-binding protein (AFABP) are increased in chronic haemodialysis (CD). Clin Endocrinol 69:901–905. https://doi.org/10.1111/j.1365-2265.2008.03277.x
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
(2013) Chapter 2: definition, identification, and prediction of CKD progression. Kidney Int Suppl 3: 63–72
Ong KL, Rye KA, O’Connell R et al (2012) Long-term fenofibrate therapy increases fibroblast growth factor 21 and retinol-binding protein 4 in subjects with type 2 diabetes. J Clin Endocrinol Metab 97:4701–4708. https://doi.org/10.1210/jc.2012-2267
Hao Y, Ma X, Luo Y et al (2014) Serum adipocyte fatty acid binding protein levels are positively associated with subclinical atherosclerosis in Chinese pre- and postmenopausal women with normal glucose tolerance. J Clin Endocrinol Metab 99:4321–4327. https://doi.org/10.1210/jc.2014-1832
Neal B, Perkovic V, Mahaffey KW et al (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:644–657. https://doi.org/10.1056/NEJMoa1611925
Niewczas MA, Gohda T, Skupien J et al (2012) Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23:507–515. https://doi.org/10.1681/ASN.2011060627
Berhane AM, Weil EJ, Knowler WC, Nelson RG, Hanson RL (2011) Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol 6:2444–2451. https://doi.org/10.2215/CJN.00580111
Ebert T, Hopf LM, Wurst U et al (2014) Circulating adipocyte fatty acid binding protein is increased in chronic and acute renal dysfunction. Nutr Metab Cardiovasc Dis 24:1027–1034. https://doi.org/10.1016/j.numecd.2014.03.006
Van JA, Scholey JW, Konvalinka A (2017) Insights into diabetic kidney disease using urinary proteomics and bioinformatics. J Am Soc Nephrol 28:1050–1061. https://doi.org/10.1681/ASN.2016091018
Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ (2006) Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) 11:226–231. https://doi.org/10.1111/j.1440-1797.2006.00576.x
Navarro JF, Milena FJ, Mora C, Leon C, Garcia J (2006) Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol 26:562–570. https://doi.org/10.1159/000098004
Wada T, Furuichi K, Sakai N et al (2000) Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 58:1492–1499. https://doi.org/10.1046/j.1523-1755.2000.00311.x
Kwok KH, Cheng KK, Hoo RL, Ye D, Xu A, Lam KS (2016) Adipose-specific inactivation of JNK alleviates atherosclerosis in apoE-deficient mice. Clin Sci (Lond) 130:2087–2100. https://doi.org/10.1042/CS20160465
Eklund CM (2009) Proinflammatory cytokines in CRP baseline regulation. Adv Clin Chem 48:111–136
Tanaka M, Furuhashi M, Okazaki Y et al (2014) Ectopic expression of fatty acid-binding protein 4 in the glomerulus is associated with proteinuria and renal dysfunction. Nephron Clin Pract 128:345–351. https://doi.org/10.1159/000368412
Yao F, Li Z, Ehara T et al (2015) Fatty acid-binding protein 4 mediates apoptosis via endoplasmic reticulum stress in mesangial cells of diabetic nephropathy. Mol Cell Endocrinol 411:232–242. https://doi.org/10.1016/j.mce.2015.05.003
Perkovic V, Zeeuw D, Mahaffey KW et al (2018) Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 6:691–704. https://doi.org/10.1016/S2213-8587(18)30141-4
Gregg EW, Cheng YJ, Srinivasan M et al (2018) Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 391:2430–2440. https://doi.org/10.1016/S0140-6736(18)30314-3
Acknowledgements
We thank RLC Wong (Department of Medicine, University of Hong Kong, Hong Kong) for her technical assistance in the measurements of serum AFABP and hsCRP levels. Some of the data were presented as an abstract at the International Diabetes Federation Western Pacific Region Congress (IDF-WPR) in 2014.
Funding
This work was supported by the Health and Medical Research Fund (reference 14150781).
Author information
Authors and Affiliations
Contributions
CHL contributed to analysis of the data and writing of the manuscript. CYYC, YCW, DTWL, MMAY, WSC and AX contributed to the interpretation of data and revising the manuscript. CHYF contributed to analysis of the data and writing of the manuscript. KSLL initiated and supervised the study, critically revised for important intellectual content and is the guarantor of this work, and as such has had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have approved the final version.
Corresponding author
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Electronic supplementary material
ESM
(PDF 184 kb)
Rights and permissions
About this article
Cite this article
Lee, C.H., Cheung, C.Y.Y., Woo, Y.C. et al. Prospective associations of circulating adipocyte fatty acid-binding protein levels with risks of renal outcomes and mortality in type 2 diabetes. Diabetologia 62, 169–177 (2019). https://doi.org/10.1007/s00125-018-4742-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4742-8