Abstract
Aims/hypothesis
The aim of this study was to assess the potential dose-dependent effects of smoking on the risk of CHD, heart failure and stroke in individuals with type 1 diabetes.
Methods
The study included 4506 individuals with type 1 diabetes who were participating in the Finnish Diabetic Nephropathy (FinnDiane) study. Intensity of smoking was estimated by packs per day and cumulative smoking by pack-years. Cox regression analyses were used to estimate the risk of incident CHD, heart failure or stroke during follow-up.
Results
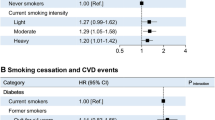
One pack per day significantly increased the risk of incident CHD in current smokers compared with never smokers (HR 1.45 [95% CI 1.15, 1.84]), after adjustment for age, sex, HbA1c, hypertension, duration of diabetes and BMI. The risk of CHD in former smokers was similar to the risk in never smokers. The risk of incident heart failure was 1.43 (95% CI 1.03, 1.97) in current smokers per one pack per day and 1.37 (95% CI 1.05, 1.77) in former smokers, while the risk of incident stroke was 1.70 (95% CI 1.26, 2.29) and 1.49 (95% CI 1.14, 1.93), respectively. After further adjustments for lipids, however, the difference in the risk of heart failure in current and former smokers was no longer significant. Cumulative smoking data were similar to smoking intensity data.
Conclusions/interpretation
There is a dose-dependent association between smoking and cardiovascular disease in individuals with type 1 diabetes. In men in particular, the risk of incident stroke remains high even after smoking cessation and is increased in current and former smokers independently of other risk factors.
Similar content being viewed by others

Introduction
Smoking is a major modifiable risk factor for cardiovascular morbidity and mortality among individuals with diabetes [1]. According to a study on diabetes care conducted in the USA between 1999 and 2010, the control of modifiable risk factors among individuals with diabetes has generally improved [2]. However, based on the same study, this is not the case with smoking; the prevalence of current smokers is still high, exceeding 20%, and has not changed during the last 10 years. A recent meta-analysis concluded that smoking is associated with an increased risk of all-cause mortality and cardiovascular events among individuals with diabetes [3]. However, most previous studies on this topic have included individuals with type 2 diabetes, and only a few have concentrated on individuals with type 1 diabetes.
The results of the available studies on the risk of CHD in individuals with type 1 diabetes have been conflicting, and most have not specifically assessed the risk of CHD but have instead addressed overall cardiovascular mortality or the combined risk of major outcomes [4,5,6,7,8,9,10,11,12]. In the general population, smoking is considered to be a risk factor for heart failure [13]. However, studies regarding the risk of incident heart failure in individuals with type 1 diabetes are scarce, and the two studies available reported contradictory findings regarding the effect of smoking on the risk of heart failure [14, 15]. Current smoking is associated with an increased risk of both ischaemic and haemorrhagic stroke in the general population [16, 17]. However, studies including only individuals with type 1 diabetes have not been able to show a similar association [7, 9, 18].
Although smoking is recognised as an important risk factor for cardiovascular disease (CVD) in the general population, the findings in individuals with type 1 diabetes have been conflicting and, therefore, the potential excess risk of CVD among individuals with type 1 diabetes who smoke has still to be proven. Of note, previous studies have often combined former smokers with either never smokers or current smokers, despite several studies showing that the risk of CVD is attenuated after smoking cessation [19,20,21].
Furthermore, all previous studies in individuals with type 1 diabetes have lacked dose–response analyses. In recent years, epidemiologists have debated the best way to quantify smoking as a risk factor for CVD [22]. Traditionally, exposure to smoking has been calculated in pack-years [23, 24]. However, a recent study suggested that the best method is to use the intensity of smoking in packs per day [25]. Given the obvious gap in quantifying the exposure to smoking in individuals with type 1 diabetes, we decided to study the association between CVD outcomes and smoking in individuals with type 1 diabetes using both intensity of smoking in packs per day and cumulative smoking in pack-years as measures of smoking exposure.
Methods
The study is part of the Finnish Diabetic Nephropathy (FinnDiane) study, an ongoing, nationwide, multicentre study that is aiming at identifying genetic and environmental risk factors for diabetic micro- and macrovascular complications. All five university hospitals, all 16 central hospitals, most regional hospitals and several major healthcare centres in Finland took part in the recruitment and characterisation of participants for the FinnDiane study. Individuals with type 1 diabetes were recruited at their regular outpatient visits. Recruitment started in 1994 and all participants who had been enrolled by the end of 2013 were included in the present study. For the current study, we analysed data from 4506 participants. Exclusion criteria were missing or unclear data on baseline smoking status (n = 261) and unclear follow-up data regarding CVD (n = 4). The entire FinnDiane protocol has previously been described in detail [26]. All participants gave written informed consent. The study protocol was in accordance with the principles of the Declaration of Helsinki as revised in 2000 and was approved by the ethics committee of Helsinki and Uusimaa Hospital District.
Lifestyle factors were assessed at the baseline visit using questionnaires, which included questions on current and former smoking habits. Participants smoking at least one cigarette per day (CPD) for at least 1 year were considered smokers, and participants who had stopped smoking before the baseline visit were considered former smokers. Never smokers were only participants who had never smoked during their lifetime. Smoking habits were also checked at any later prospective visits (data available for 1566 participants) as well as through a follow-up questionnaire that was mailed to participants in 2015 (data available for 1903 participants). Based on this additional information, missing baseline smoking status was reconstructed for 118 participants (2.6%) and corrected for 46 participants (1.0%). Smoking intensity was calculated using packs (i.e. 20 cigarettes) per day. Cumulative smoking was measured using pack-years, where 1 pack-year equals smoking 20 CPD during 1 year.
Participants underwent a thorough physical examination, and blood and urine samples were collected. Participants were considered to have hypertension if the mean BP was ≥140/90 mmHg or they were taking antihypertensive medication. BMI was calculated as weight (kg) divided by the square of height (m). Baseline diabetic nephropathy was defined as either macroalbuminuria (urinary albumin excretion rate ≥200 μg/min or ≥300 mg/24 h) or end-stage renal disease (dialysis or kidney transplant). Participants were divided into six different social classes based on their education and occupational status.
Follow-up data for incident cardiovascular events were based on the Finnish Care Register for Health Care or Cause of Death Register. The following International Classification of Diseases (ICD) and other codes were used for CHD: myocardial infarction (ICD-8/9 [www.icd9data.com/2007/Volume1] 410, ICD-10 [http://apps.who.int/classifications/icd10/browse/2016/en] I21–I22) or coronary intervention codes for coronary artery bypass surgery or balloon angioplasty (procedure codes based on the Nordic Medico-Statistical Committee [NOMESCO] since 1996 http://nordclass.se/ncsp_e.htm and https://thl.fi/fi/web/kansantaudit/sydan-ja-verisuonitaudit/sydan-ja-verisuonitautirekisteri, accessed 12 August 2018: TFN40, FN1AT, FN1BT, FN1YT [all from the Finnish version of the NOMESCO codes], FNF, FNG, FNA, FNB, FNC, FND, FNE; and surgical procedure codes according to the procedure classification of the Finnish Hospital Association 1983-1995 [27]: 5311–5315); heart failure (ICD-8 4270, 4271, 7824, ICD-9 4280–4289, ICD-10 I50); and ischaemic/haemorrhagic stroke (ICD-8/9430–434, ICD-10 I60–I64). All participants without an existing condition of the diagnosis in question were included in the analyses. Follow-up started at the baseline visit and ended with a diagnosis of CHD, heart failure or stroke, death from any cause or the end of year 2014.
Statistical analyses
Based on smoking status, the participants were divided into three groups: never, current and former smokers. Data on baseline characteristics are presented as means ± SD for normally distributed values, and otherwise as medians (interquartile range [IQR]). Categorical variables are reported as percentages. Differences between groups were analysed using ANOVA for normally distributed continuous variables, and otherwise using the Kruskal–Wallis test. Differences between categorical variables were analysed using the χ2 test.
The effects of smoking status, intensity of smoking and cumulative smoking were analysed using Cox regression models, providing HRs with 95% CIs for the development of CHD, heart failure and combined ischaemic or haemorrhagic stroke. Never smokers were used as the reference group and smoking status was used as a categorical variable. Non-linearity of the effects of packs per day and pack-years on CVD outcomes were tested using restricted cubic spline models (electronic supplementary material [ESM] Figs 1, 2). Packs per day and pack-years were used both as continuous variables and as dichotomised categorical variables.
Possible confounding factors were included in four different models. In the first model, the results were adjusted for age and sex. In the second model, social class (the two highest classes compared with the others) and baseline alcohol intake (yes/no) were also included as environmental risk factors. In the third and fourth models, the results were adjusted for traditional risk factors for CVD: HbA1c, hypertension, duration of diabetes, BMI, HDL-cholesterol and loge triacylglycerol. If the results were significant in the fourth model, the presence of diabetic nephropathy was added into the model. Interaction terms between sex and packs per day and pack-years were entered into the models and, in the case of no interaction, men and women were pooled; otherwise, the modelling was performed separately for men and women. Finally, to verify our findings, we performed a meta-analysis with three previous studies [7, 9, 10] that had reported risk estimates for CHD or stroke in individuals with type 1 diabetes based on smoking status. We used odds ratios or hazard ratios to calculate summary measures. Meta-analyses were conducted by using random-effects models by the %METAANAL SAS macro [28].
Findings were considered statistically significant at p < 0.05. IBM SPSS statistics version 24 (IBM Corporation, Armonk, NY, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA) were used for all statistical analyses.
Results
Risk of CVD based on smoking status
Participants’ baseline characteristics are shown in Table 1. The participants were divided based on smoking status as follows: never smokers 2335 (51.8%), current smokers 1147 (25.5%) and former smokers 1024 (22.7%). The proportion of men was higher among current and former smokers compared with never smokers. Current smokers had shorter duration of diabetes, lower BMI and poorer HbA1c and lipid values compared with never smokers, but there were no differences in BPs. Former smokers were older, had a longer duration of diabetes, higher BP and poorer lipid values compared with never smokers. The proportion of participants in the two highest social classes was lower among current and former smokers than among never smokers. Higher proportions of current and former smokers vs never smokers also consumed alcohol.
The overall follow-up time for CHD was 53,062 person-years, with a median follow-up of 13.6 (IQR 10.6–15.7) years. Corresponding follow-up times were 55,856 person-years for heart failure, with a median of 13.8 (IQR 10.9–15.8) years, and 54,518 person-years for any stroke, with a median of 13.7 (IQR 10.8–15.8) years. During follow-up, there were 512 incident CHD, 313 heart failure, 304 any stroke, 240 ischaemic and 83 haemorrhagic stroke events.
Table 2 presents the cumulative risk of CVD based on baseline smoking status. Current smokers had a higher risk of CHD with an HR of 1.35 (95% CI 1.08, 1.69), but the risk of CHD in former smokers was similar to that of never smokers. In contrast, the risk of heart failure was higher in both current smokers (HR 1.42 [95% CI 1.05, 1.93]) and former smokers (HR 1.35 [95% CI 1.04, 1.76]). However, after further adjustments for lipids, the risk of CHD in current smokers and the risk of heart failure in current and former smokers was attenuated and the difference compared with never smokers was no longer significant. There was an interaction between sex and smoking status regarding the risk of any stroke (p = 0.02) and the data were analysed separately for men and women. In men, both current and former smokers had a higher risk of any stroke compared with never smokers, and the difference was significant even after baseline diabetic nephropathy status was added into the final model (current smokers: HR 1.90 [95% CI 1.29, 2.82]; former smokers: HR 1.92 [95% CI 1.32, 2.80]; data not shown). In women, there were no significant differences between current/former and never smokers regarding the risk of stroke.
The effect of smoking on risk of ischaemic and haemorrhagic stroke
ESM Table 1 shows the results of Cox regression analyses for the risk of ischaemic and haemorrhagic stroke. There was an interaction between sex and smoking status regarding ischaemic stroke (p = 0.008) and the results are shown separately for men and women. In men, the risk of ischaemic stroke was higher for both current and former smokers in all multivariable models and also after baseline diabetic nephropathy status was added into the model (current smokers: HR 2.10 [95% CI 1.33, 3.32]; former smokers: HR 2.40 [95% CI 1.57, 3.67]; data not shown). Again, in women, the risk of ischaemic stroke was similar in all groups. In men and women combined, the risk of haemorrhagic stroke was elevated among current smokers after adjustment for age, sex, social class and alcohol intake (HR 1.86 [95% CI 1.06, 3.27]), but adjustments for other CVD risk factors diluted the results and the difference between current and never smokers was no longer significant.
Risk of CVD based on intensity of smoking (packs per day)
Table 3 presents the results of Cox regression analyses for the risk of CHD, heart failure and combined ischaemic and haemorrhagic stroke based on the intensity of smoking in packs per day. Current smokers had a higher risk of CHD compared with never smokers, with an HR of 1.28 (95% CI 1.00, 1.63) per one pack per day, adjusted for age, sex, HbA1c, hypertension, duration of diabetes, BMI, HDL-cholesterol and loge triacylglycerol. However, when the presence of nephropathy was included in the model, the difference was no longer significant (HR 1.20 [95% CI 0.93, 1.54]). In former smokers, the risk of incident CHD was higher compared with never smokers, with an HR of 1.25 (95% CI 1.00, 1.56) when the results were adjusted for age and sex. However, the results were attenuated after adjustment for other CVD risk factors and the difference was no longer significant.
One pack per day increased the risk of heart failure in current smokers (HR 1.43 [95% CI 1.03, 1.97]) and former smokers (HR 1.37 [95% CI 1.05, 1.77]), after adjustments for age, sex, HbA1c, hypertension, duration of diabetes and BMI. However, when the lipid variables were included in the model, the differences were no longer significant.
Regarding the risk of any stroke, there were no interactions between sex and packs per day or pack-years, and therefore men and women were analysed together. The risk of combined ischaemic and haemorrhagic stroke was increased in both former and current smokers, with one pack per day increasing the risk of any stroke with an HR of 1.41 (95% CI 1.02, 1.93) in current smokers and 1.36 (95% CI 1.03, 1.81) in former smokers in the final model, including baseline nephropathy status (data not shown).
Risk of CVD based on cumulative smoking (pack-years)
Table 4 shows the results for the risk of different CVD outcomes based on cumulative smoking in pack-years. The results were similar to those of the analyses regarding intensity of smoking in packs per day. Compared with never smokers, the risks of CHD in current smokers and of heart failure in former smokers were significantly higher after the lipid variables were included in the model, but no longer significant after adjustment for baseline diabetic nephropathy (data not shown). However, the risk of any stroke remained significantly increased compared with never smokers in both former and current smokers in all models, even after adjustment for nephropathy status, and one pack-year increased the risk of any stroke with an HR of 1.013 (95% CI 1.001, 1.025) in current smokers 1.012 (95% CI 1.001, 1.024) in former smokers (data not shown).
ESM Tables 2 and 3 present the results of CVD risk in dichotomised groups of intensity of smoking and cumulative smoking compared with never smokers. The results were similar to those found with the continuous data and, in current smokers, the CVD risk was highest in the groups of higher intensity and longer cumulative smoking. Among former smokers who had smoked ≥20 pack-years, the risk of CHD was similar to that of never smokers (HR 1.06 [95% CI 0.73, 1.54]), although current smokers with the same cumulative dose of smoking had an increased risk of CHD (HR 1.52 [95% CI 1.12, 2.06]).
Table 5 presents the results of the meta-analysis. The study by Klein et al reported combined results, while the other two studies separate results for men and women [7, 9, 10]. Compared with never smokers, the risk of CHD was increased in current smokers (RR 1.36 [95% CI 1.07, 1.72]), while the risk of any stroke was increased in men who were former smokers (RR 1.77 [95% CI 1.00, 3.14]) or current smokers (RR 2.13 [95% CI 1.52, 3.00]).
Discussion
In this study in participants with type 1 diabetes, we identified novel dose-dependent associations between smoking and cardiovascular outcomes. We observed an increased risk of CHD in current smokers, both associated with the intensity of smoking in packs per day and with cumulative smoking in pack-years. Similar dose-dependent associations were also seen in the risk of heart failure. Furthermore, in men, the risk of combined ischaemic and haemorrhagic stroke was elevated in current smokers and the risk was not significantly reduced after smoking cessation.
When our results are compared with studies from the general population, there are some differences. A recent large meta-analysis reported a higher risk of acute coronary events in current smokers of ≥20 CPD compared with never smokers, with an HR of 2.43 (95% CI 2.01, 2.93) [29], which is higher than our HR for CHD of 1.34–1.62 in current smokers of ≥20 CPD (ESM Table 2) [29]. This could be explained by the fact that diabetes already causes a three- to fivefold increase in the risk of CHD, and therefore the effect of smoking is diluted in a study that includes only people with diabetes. The HR for stroke in the same meta-analysis was 1.91 (95% CI 1.66, 2.21) [29], which is similar to the excess risk in men observed in our study. In the Health, Aging and Body Composition (Health ABC) Study, the risk of heart failure was increased in current smokers vs non-smokers (HR 1.73 [95% CI 1.15, 2.59]) [13], which is higher than the effect seen in our study. However, their results for former smokers were comparable with ours.
Although smoking is an established cardiovascular risk factor in the general population, surprisingly few studies have shown an increased risk of CHD in individuals with type 1 diabetes who are current smokers. In the Wisconsin Epidemiologic Study of Diabetic Retinopathy, both current and past smoking were associated with an increased risk of myocardial infarction, but not with stroke [9]. In The Pittsburgh Epidemiology of Diabetes Complications Study, combined ever smoking was an independent risk factor for total coronary artery disease and angina, but smoking was not associated with ‘hard’ coronary artery disease events [8]. In the EURODIAB Prospective Complications Study, smoking was associated with CHD in men but not in women [10]. It is of note that in our study, when smoking status (yes/no) was used as the variable, the risk of CHD in current smokers was similar to that in never smokers after adjustment for other CVD risk factors, including lipids. This is in line with the CHD results of the WHO Multinational Study of Vascular Disease in Diabetes [7].
However, when smoking exposure was measured in packs per day or pack-years, current smokers indeed had a higher risk of CHD compared with never smokers, even after adjustment for other CVD risk factors, including lipids, but no longer after adjustment for the presence of diabetic nephropathy. This is no surprise, since we have previously demonstrated that cumulative smoking is a risk factor for diabetic nephropathy [30], and it is well known that individuals with diabetic nephropathy carry a markedly increased risk of CVD compared with those without nephropathy [31]. Therefore, diabetic nephropathy could rather be considered a mediating factor, than a confounding factor, when the association between smoking and CVD is analysed. Similarly, smoking also affects other traditional cardiovascular risk factors, such as lipids and glycaemic control [32,33,34].
In the present study, the risk of heart failure was increased in both former and current smokers compared with never smokers, and this risk increased with increasing intensity and cumulative dose of smoking. These results for current smokers are in line with those of a previous Swedish study regarding heart failure in individuals with type 1 diabetes [14]. Another Polish study did not observe an independent association between smoking and heart failure [15]. In both studies, however, the definition of smokers was not clearly reported and the risk of former smokers was not separately analysed. Notably, the results regarding the risk of heart failure in former and current smokers are similar to those regarding the risk of CHD in current smokers. This is not surprising and could partly be explained by the fact that CHD is the most common underlying cause of heart failure [35].
Our data also show that, in men, both current and former smokers carry an increased risk of stroke. It is of note that the harmful effect of smoking on the development of stroke seems to be independent of other traditional CVD risk factors and the presence of diabetic nephropathy, unlike the effect on CHD and heart failure. These results are in contrast to those from the WHO Multinational Study of Vascular Disease in Diabetes and the Wisconsin Epidemiologic Study of Diabetic Retinopathy [7, 9]. However, these divergent results can be explained by a lack of statistical power in those two studies, caused by the small number of stroke events. In the Pittsburgh Epidemiology of Diabetes Complications study, ever smoking was a major predictor of ischaemic stroke, although it was no longer significant after the results were adjusted for other cardiovascular risk factors and diabetic nephropathy [18]. In our study, the risk of stroke was increased in current and former smokers irrespective of the baseline nephropathy status, but only in men. In the previous studies men and women were studied together and that, combined with smaller cohort sizes, could explain the different results compared with our study. As expected, our results are in line with previously reported data from the FinnDiane study [36]. However, this time we analysed former and current smokers separately, and we could show that the risk of stroke increased with increasing intensity of smoking and increased cumulative dose of smoking.
In women, we were unable to observe an association between smoking status and the risk of stroke. However, regarding the risk of stroke, there was no interaction between sex and intensity of smoking or cumulative smoking, showing that the dose-dependent effect of smoking is similar in men and women, although the results from packs per day and pack-year analyses refer more to the risk seen in men, because of the larger number of men who were current and former smokers.
In the subgroup analyses, we observed that the effect of smoking was clearly stronger on the risk of ischaemic stroke. Again, this was only seen in men. Furthermore, an increased risk of haemorrhagic stroke was seen only in current smokers, but this risk was attenuated after adjusting for other CVD risk factors. This could, of course, be due to the rather small number of haemorrhagic stroke events in our cohort, and therefore it is not clear if current smoking is indeed associated with an increased risk of haemorrhagic stroke in individuals with type 1 diabetes.
Finally, we meta-analysed our results with those of previous reports regarding cardiovascular events in individuals with type 1 diabetes. The results were in line with our data, showing an increased risk of CHD in current smokers and an increased risk of stroke in men who were current or former smokers.
There are a number of limitations to the present study. Smoking variables were based on self-reported questionnaires and there were no laboratory measurements, such as urinary cotinine levels. Furthermore, people underestimate rather than overestimate their real consumption of cigarettes, a fact that could have influenced the dose-dependent analyses. Some of the current and former smokers were excluded from the dose-dependent analyses because of missing data on number of smoked cigarettes (83 participants) or time of exposure (252 participants). This might have affected the pack-year analyses. Recruitment of participants started in 1994 and ended in 2013, a fact that might have led to temporal effects. Based on the FinnDiane data, the proportion of current smokers declined during this period from 30% to less than 20%. At the same time, the care of individuals with acute coronary syndrome has improved. These factors might have impacted the associations between smoking and cardiovascular mortality. However, we studied the overall incidence of different CVD events, and instead of using just smoking status, we assessed the effects of smoking intensity and cumulative smoking. A further limitation of our study is the lack of data on time-varying exposure of smoking during follow-up. Although we had follow-up data regarding smoking behaviour for around 2000 participants, we did not have the complete data on smoking history before each cardiovascular event for every participant, and therefore the total exposure during follow-up was not calculated and only the intensity of smoking and retrograde cumulative smoking were used. This could diminish the effect of cumulative smoking, especially in younger participants, who were recruited during the earlier years.
The analyses regarding the intensity of smoking (packs per day) and cumulative smoking (pack-years) gave similar results and, based on these results, both measures seem to perform rather well when estimating the excess risk of CVD in current or former smokers with type 1 diabetes. However, information on smoking intensity is more often available than that on pack-years, and it therefore appears that the smoking intensity could be a better measure of smoking exposure. Nevertheless, the packs per day and pack-year variables seem to be superior to smoking status, at least when addressing the risk of CHD in smokers.
To our knowledge, this is the first study to demonstrate a dose-dependent effect of smoking on the risk of CHD, heart failure and stroke in individuals with type 1 diabetes. Individuals with type 1 diabetes already have a significantly elevated risk of CHD, and this risk is further increased by smoking. In men, the effect of smoking seems to be not only harmful but also persistent regarding the development of ischaemic stroke, with this risk remaining even after smoking cessation.
Data availability
Data regarding the additional analysis are available by request. The ethical statement and the informed consent do not allow the data to be freely available.
Abbreviations
- CPD:
-
Cigarettes per day
- CVD:
-
Cardiovascular disease
- FinnDiane :
-
Finnish Diabetic Nephropathy study
- IQR:
-
Interquartile range
References
Vazquez-Benitez G, Desai JR, Xu S et al (2015) Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care 38:905–912
Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW (2013) Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 368:1613–1624
Pan A, Wang Y, Talaei M, Hu FB (2015) Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation 132:1795–1804
Moy CS, LaPorte RE, Dorman JS et al (1990) Insulin-dependent diabetes mellitus mortality. The risk of cigarette smoking. Circulation 82:37–43
Rossing P, Hougaard P, Borch-Johnsen K, Parving HH (1996) Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. BMJ 313:779–784
Chaturvedi N, Stevens L, Fuller JH (1997) Which features of smoking determine mortality risk in former cigarette smokers with diabetes? The World Health Organization Multinational Study Group. Diabetes Care 20:1266–1272
Fuller JH, Stevens LK, Wang SL (2001) Risk factors for cardiovascular mortality and morbidity: the WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia 44(Suppl 2):S54–S64
Orchard TJ, Olson JC, Erbey JR et al (2003) Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 26:1374–1379
Klein BE, Klein R, McBride PE et al (2004) Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med 164:1917–1924
Soedamah-Muthu SS, Chaturvedi N, Toeller M et al (2004) Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB prospective complications study. Diabetes Care 27:530–537
Miller RG, Prince CT, Klein R, Orchard TJ (2009) Retinal vessel diameter and the incidence of coronary artery disease in type 1 diabetes. Am J Ophthalmol 147:653–660
Miller RG, Secrest AM, Ellis D, Becker DJ, Orchard TJ (2013) Changing impact of modifiable risk factors on the incidence of major outcomes of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 36:3999–4006
Gopal DM, Kalogeropoulos AP, Georgiopoulou VV et al (2012) Cigarette smoking exposure and heart failure risk in older adults: the health, aging, and body composition study. Am Heart J 164:236–242
Lind M, Bounias I, Olsson M, Gudbjornsdottir S, Svensson AM, Rosengren A (2011) Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 378:140–146
Konduracka E, Cieslik G, Galicka-Latala D et al (2013) Myocardial dysfunction and chronic heart failure in patients with long-lasting type 1 diabetes: a 7-year prospective cohort study. Acta Diabetol 50:597–606
Woodward M, Lam TH, Barzi F et al (2005) Smoking, quitting, and the risk of cardiovascular disease among women and men in the Asia-Pacific region. Int J Epidemiol 34:1036–1045
O'Donnell MJ, Xavier D, Liu L et al (2010) Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 376:112–123
Secrest AM, Prince CT, Costacou T, Miller RG, Orchard TJ (2013) Predictors of and survival after incident stroke in type 1 diabetes. Diab Vasc Dis Res 10:3–10
Lightwood JM, Glantz SA (1997) Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation 96:1089–1096
Critchley JA, Capewell S (2003) Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 290:86–97
Jha P, Ramasundarahettige C, Landsman V et al (2013) 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 368:341–350
Thomas DC (2014) Invited commentary: is it time to retire the “pack-years” variable? Maybe not. Am J Epidemiol 179:299–302
Lubin JH, Couper D, Lutsey PL, Woodward M, Yatsuya H, Huxley RR (2016) Risk of cardiovascular disease from cumulative cigarette use and the impact of smoking intensity. Epidemiology 27:395–404
McEvoy JW, Blaha MJ, DeFilippis AP et al (2015) Cigarette smoking and cardiovascular events: role of inflammation and subclinical atherosclerosis from the MultiEthnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 35:700–709
Nance R, Delaney J, McEvoy JW et al (2017) Smoking intensity (pack/day) is a better measure than pack-years or smoking status for modeling cardiovascular disease outcomes. J Clin Epidemiol 81:111–119
Thorn LM, Forsblom C, Fagerudd J et al (2005) Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 28:2019–2024
Finnish Hospital league (1983) Toimenpidenimikkeistö 1983. (Classification of procedures 1983, in Finnish). Finnish Hospital League, Helsinki
Hertzmark E, Spiegelman D. 2017 The SAS METAANAL Macro. Available at https://www.hsph.harvard.edu/donna-spiegelman/software/metaanal/
Mons U, Muezzinler A, Gellert C et al (2015) Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ 350:h1551
Feodoroff M, Harjutsalo V, Forsblom C et al (2016) Smoking and progression of diabetic nephropathy in patients with type 1 diabetes. Acta Diabetol 53:525–533
Borch-Johnsen K, Kreiner S (1987) Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 294:1651–1654
Fisher SD, Zareba W, Moss AJ et al (2000) Effect of smoking on lipid and thrombogenic factors two months after acute myocardial infarction. Am J Cardiol 86:813–818
Craig WY, Palomaki GE, Haddow JE (1989) Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ 298:784–788
Gerber PA, Locher R, Schmid B, Spinas GA, Lehmann R (2013) Smoking is associated with impaired long-term glucose metabolism in patients with type 1 diabetes mellitus. Nutr Metab Cardiovasc Dis 23:102–108
McMurray JJ, Stewart S (2000) Epidemiology, aetiology, and prognosis of heart failure. Heart 83:596–602
Hagg S, Thorn LM, Forsblom CM et al (2014) Different risk factor profiles for ischemic and hemorrhagic stroke in type 1 diabetes mellitus. Stroke 45:2558–2562
Acknowledgements
The authors would like to acknowledge all of the physicians and nurses at each FinnDiane centre participating in the recruitment and characterisation of participants (see ESM).
Funding
This research was supported by grants from the Folkhälsan Research Foundation, Academy of Finland (134379), Wilhelm and Else Stockmann Foundation, Diabetes Research Foundation, Liv och Hälsa Foundation, Finska Läkaresällskapet, Novo Nordisk Foundation, Signe and Ane Gyllenberg Foundation, and Päivikki and Sakari Sohlberg Foundation. The funding sources were not involved in the design or conduct of the study.
Author information
Authors and Affiliations
Consortia
Contributions
MF designed the study, carried out the statistical analyses and wrote the manuscript. VH contributed to the design of the study, statistical analyses and critically revised the manuscript. CF contributed to the design of the study and critically revised the manuscript. P-HG contributed to the study design, and edited and critically revised the manuscript. P-HG is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
P-HG has received investigator-initiated research grants from Eli Lilly and Roche, is an advisory board member for AbbVie, Astra Zeneca, Boehringer-Ingelheim, Eli Lilly, Janssen, Medscape, MSD, Novartis, Novo Nordisk and Sanofi, and has been an advisory board member for Cebix. He has received lecture fees from Astra Zeneca, Boehringer-Ingelheim, Eli Lilly, ELO Water, Genzyme, Medscape, MSD, Novartis, Novo Nordisk and Sanofi. All other authors declare that there is no duality of interest associated with this manuscript.
Electronic supplementary material
ESM
(PDF 1267 kb)
Rights and permissions
About this article
Cite this article
Feodoroff, M., Harjutsalo, V., Forsblom, C. et al. Dose-dependent effect of smoking on risk of coronary heart disease, heart failure and stroke in individuals with type 1 diabetes. Diabetologia 61, 2580–2589 (2018). https://doi.org/10.1007/s00125-018-4725-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4725-9