Abstract
Aims/hypothesis
Research on the pathogenesis of type 1 diabetes relies heavily on good animal models. The aim of this work was to study the translational value of animal models of type 1 diabetes to the human situation.
Methods
We compared the four major animal models of spontaneous type 1 diabetes, namely the NOD mouse, BioBreeding (BB) rat, Komeda rat and LEW.1AR1-iddm rat, by examining the immunohistochemistry and in situ RT-PCR of immune cell infiltrate and cytokine pattern in pancreatic islets, and by comparing findings with human data.
Results
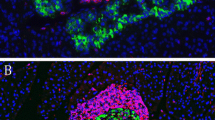
After type 1 diabetes manifestation CD8+ T cells, CD68+ macrophages and CD4+ T cells were observed as the main immune cell types with declining frequency, in infiltrated islets of all diabetic pancreases. IL-1β and TNF-α were the main proinflammatory cytokines in the immune cell infiltrate in NOD mice, BB rats and LEW.1AR1-iddm rats, as well as in humans. The Komeda rat was the exception, with IFN-γ and TNF-α being the main cytokines. In addition, IL-17 and IL-6 and the anti-inflammatory cytokines IL-4, IL-10 and IL-13 were found in some infiltrating immune cells. Apoptotic as well as proliferating beta cells were observed in infiltrated islets. In healthy pancreases no proinflammatory cytokine expression was observed.
Conclusions/interpretation
With the exception of the Komeda rat, the animal models mirror very well the situation in humans with type 1 diabetes. Thus animal models of type 1 diabetes can provide meaningful information on the disease processes in the pancreas of patients with type 1 diabetes.



Similar content being viewed by others
Abbreviations
- BB:
-
BioBreeding
- DIG:
-
Digoxigenin
- FoxP3:
-
Forkhead box P3
- iNOS:
-
Inducible nitric oxide synthase
- NFκB:
-
Nuclear factor κB
- TGF-β1:
-
Transforming growth factor β1
References
Imagawa A, Hanafusa T, Itoh N et al (1996) Islet-infiltrating T lymphocytes in insulin-dependent diabetic patients express CD80 (B7-1) and CD86 (B7-2). J Autoimmun 9:391–396
Imagawa A, Hanafusa T, Tamura S et al (2001) Pancreatic biopsy as a procedure for detecting in situ autoimmune phenomena in type 1 diabetes: close correlation between serological markers and histological evidence of cellular autoimmunity. Diabetes 50:1269–1273
Uno S, Imagawa A, Okita K et al (2007) Macrophages and dendritic cells infiltrating islets with or without beta cells produce tumour necrosis factor-alpha in patients with recent-onset type 1 diabetes. Diabetologia 50:596–601
Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y (1980) Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 29:1–13
Anderson MS, Bluestone JA (2005) The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 23:447–485
Gysemans C, Callewaert H, Moore F et al (2009) Interferon regulatory factor-1 is a key transcription factor in murine beta cells under immune attack. Diabetologia 52:2374–2384
Koulmanda M, Bhasin M, Awdeh Z et al (2012) The role of TNF-alpha in mice with type 1- and 2- diabetes. PLoS One 7:e33254
Nakhooda AF, Like AA, Chappel CI, Murray FT, Marliss EB (1977) The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes 26:100–112
Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL (2004) Rat models of type 1 diabetes: genetics, environment, and autoimmunity. ILAR J 45:278–291
Wang GS, Kauri LM, Patrick C, Bareggi M, Rosenberg L, Scott FW (2010) Enhanced islet expansion by beta-cell proliferation in young diabetes-prone rats fed a protective diet. J Cell Physiol 224:501–508
Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T (1991) New inbred strain of Long-Evans Tokushima lean rats with IDDM without lymphopenia. Diabetes 40:1375–1381
Komeda K, Noda M, Terao K, Kuzuya N, Kanazawa M, Kanazawa Y (1998) Establishment of two substrains, diabetes-prone and non-diabetic, from Long-Evans Tokushima Lean (LETL) rats. Endocr J 45:737–744
Lenzen S, Tiedge M, Elsner M et al (2001) The LEW.1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia 44:1189–1196
Jörns A, Günther A, Hedrich HJ, Wedekind D, Tiedge M, Lenzen S (2005) Immune cell infiltration, cytokine expression, and beta-cell apoptosis during the development of type 1 diabetes in the spontaneously diabetic LEW.1AR1/Ztm-iddm rat. Diabetes 54:2041–2052
Arndt T, Jörns A, Weiss H et al (2013) A variable CD3(+) T cell frequency in peripheral blood lymphocytes associated with type 1 diabetes mellitus development in the LEW.1AR1-iddm rat. PLoS One 8:e64305
King AJ (2012) The use of animal models in diabetes research. Br J Pharmacol 166:877–894
Buschard K (2011) What causes type 1 diabetes? Lessons from animal models. APMIS, Suppl 132:1–19
Roep BO, Atkinson M, von Herrath M (2004) Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat Rev Immunol 4:989–997
Roep BO, Buckner J, Sawcer S, Toes R, Zipp F (2012) The problems and promises of research into human immunology and autoimmune disease. Nat Med 18:48–53
Pearson T, Greiner DL, Shultz LD (2008) Creation of “humanized” mice to study human immunity. Curr Protoc Immunol Chapter 15:Unit 15.21
Jörns A, Rath KJ, Terbish T et al (2010) Diabetes prevention by immunomodulatory FTY720 treatment in the LEW.1AR1-iddm rat despite immune cell activation. Endocrinology 151:3555–3565
MacMurray AJ, Moralejo DH, Kwitek AE et al (2002) Lymphopenia in the BB rat model of type 1 diabetes is due to a mutation in a novel immune-associated nucleotide (Ian)-related gene. Genome Res 12:1029–1039
Sommandas V, Rutledge EA, van Yserloo B, Fuller J, Lernmark A, Drexhage HA (2007) Low-density cells isolated from the rat thymus resemble branched cortical macrophages and have a reduced capability of rescuing double-positive thymocytes from apoptosis in the BB-DP rat. J Leukoc Biol 82:869–876
Atkinson MA, Bluestone JA, Eisenbarth GS et al (2011) How does type 1 diabetes develop? the notion of homicide or beta-cell suicide revisited. Diabetes 60:1370–1379
Reddy S, Chai RC, Rodrigues JA, Hsu TH, Robinson E (2008) Presence of residual beta cells and co-existing islet autoimmunity in the NOD mouse during longstanding diabetes: a combined histochemical and immunohistochemical study. J Mol Histol 39:25–36
Coppieters KT, Dotta F, Amirian N et al (2012) Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 209:51–60
Lally FJ, Ratcliff H, Bone AJ (2001) Apoptosis and disease progression in the spontaneously diabetic BB/S rat. Diabetologia 44:320–324
Mordes JP, Desemone J, Rossini AA (1987) The BB rat. Diabetes Metab Rev 3:725–750
Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK (2011) Immunopathology of the human pancreas in type-I diabetes. Semin Immunopathol 33:9–21
Rowe PA, Campbell-Thompson ML, Schatz DA, Atkinson MA (2011) The pancreas in human type 1 diabetes. Semin Immunopathol 33:29–43
Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER (2013) Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS One 8:e59701
Voorbij HA, Jeucken PH, Kabel PJ, de Haan M, Drexhage HA (1989) Dendritic cells and scavenger macrophages in pancreatic islets of prediabetic BB rats. Diabetes 38:1623–1629
Akirav EM, Baquero MT, Opare-Addo LW et al (2011) Glucose and inflammation control islet vascular density and beta-cell function in NOD mice: control of islet vasculature and vascular endothelial growth factor by glucose. Diabetes 60:876–883
Kleemann R, Scott FW, Worz-Pagenstert U, Nimal Ratnayake WM, Kolb H (1998) Impact of dietary fat on Th1/Th2 cytokine gene expression in the pancreas and gut of diabetes-prone BB rats. J Autoimmun 11:97–103
Mastrandrea L, Yu J, Behrens T et al (2009) Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care 32:1244–1249
Arif S, Cox P, Afzali B et al (2010) Anti-TNFalpha therapy–killing two birds with one stone? Lancet 375:2278
Kalden JR (2011) Anti-TNF therapy: what have we learned in 12 years? Arthritis Res Ther 13(Suppl. 1): S1
Wang F, Smith N, Maier L et al (2012) Etanercept suppresses regenerative hyperplasia in psoriasis by acutely downregulating epidermal expression of IL-19, IL-20 and IL-24. Br J Dermatol 167:92–102
Goldbach-Mansky R (2012) Immunology in clinic review series; focus on autoinflammatory diseases: update on monogenic autoinflammatory diseases: the role of interleukin (IL)-1 and an emerging role for cytokines beyond IL-1. Clin Exp Immunol 167:391–404
Ordas I, Mould DR, Feagan BG, Sandborn WJ (2012) Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 91:635–646
Yokoi N, Komeda K, Wang HY et al (2002) Cblb is a major susceptibility gene for rat type 1 diabetes mellitus. Nat Genet 31:391–394
Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A (2010) Beta-cell growth and regeneration: replication is only part of the story. Diabetes 59:2340–2348
Salpeter SJ, Klein AM, Huangfu D, Grimsby J, Dor Y (2010) Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development 137:3205–3213
Rabinovitch A, Suarez-Pinzon WL (1998) Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol 55:1139–1149
Bergmann L, Kröncke KD, Suschek C, Kolb H, Kolb-Bachofen V (1992) Cytotoxic action of IL-1 beta against pancreatic islets is mediated via nitric oxide formation and is inhibited by NG-monomethyl-L-arginine. FEBS Lett 299:103–106
Kacheva S, Lenzen S, Gurgul-Convey E (2011) Differential effects of proinflammatory cytokines on cell death and ER stress in insulin-secreting INS1E cells and the involvement of nitric oxide. Cytokine 55:195–201
Ortis F, Pirot P, Naamane N et al (2008) Induction of nuclear factor-kappaB and its downstream genes by TNF-alpha and IL-1beta has a pro-apoptotic role in pancreatic beta cells. Diabetologia 51:1213–1225
Sleater M, Diamond AS, Gill RG (2007) Islet allograft rejection by contact-dependent CD8+ T cells: perforin and FasL play alternate but obligatory roles. Am J Transplant 7:1927–1933
Barthson J, Germano CM, Moore F et al (2011) Cytokines tumor necrosis factor-alpha and interferon-gamma induce pancreatic beta-cell apoptosis through STAT1-mediated Bim protein activation. J Biol Chem 286:39632–39643
Walz M, Overbergh L, Mathieu C, Kolb H, Martin S (2002) A murine interleukin-4-Ig fusion protein regulates the expression of Th1- and Th2-specific cytokines in the pancreas of NOD mice. Horm Metab Res 34:561–569
Acknowledgements
We thank D. Lischke and U. Sommerfeld (both from the Institute of Clinical Biochemistry, Hannover Medical School) for skilful technical assistance.
Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft (JO 395/2-1), from the European Union (Collaborative Project NAIMIT in the 7th Framework Programme, Contract No. 241447) to PM, CM and SL and from the Canadian Institutes of Health Research and the Canadian Diabetes Association and Cure Diabetes to FWS. CM is a clinical researcher at the FWO-Vlaanderen.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
AJ designed and performed experiments, analysed data, and wrote manuscript. TA, AMV, JK, DW, H-JH, LM, PM, NH, YN, G-SW FWS, CG and CM contributed to acquisition of data and revised the manuscript according to the discussion section. G-SW and CM drafted the article and revised it critically for important intellectual content. SL designed experiments, contributed to the discussion and wrote the manuscript. All authors revised and approved the final version to be published.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
(PDF 51 kb)
ESM Table 2
(PDF 81 kb)
ESM Table 3
(PDF 79 kb)
ESM Table 4
(PDF 158 kb)
ESM Table 5
(PDF 86 kb)
ESM Table 6
(PDF 86 kb)
ESM Table 7
(PDF 161 kb)
Rights and permissions
About this article
Cite this article
Jörns, A., Arndt, T., zu Vilsendorf, A.M. et al. Islet infiltration, cytokine expression and beta cell death in the NOD mouse, BB rat, Komeda rat, LEW.1AR1-iddm rat and humans with type 1 diabetes. Diabetologia 57, 512–521 (2014). https://doi.org/10.1007/s00125-013-3125-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3125-4