Abstract
Aims/hypothesis
Enhanced vascular inflammation, immune cell infiltration and elevated production of reactive oxygen species (ROS) contribute significantly to pro-atherogenic responses in diabetes. We assessed the immunomodulatory role of NADPH oxidase (NOX)-derived ROS in diabetes-accelerated atherosclerosis.
Methods
Diabetes was induced in male Apoe −/− mice with five daily doses of streptozotocin (55 mg kg−1 day−1). Atherosclerotic plaque size, markers of ROS and immune cell accumulation were assessed in addition to flow cytometric analyses of cells isolated from the adjacent mediastinal lymph nodes (meLNs). The role of NOX-derived ROS was investigated using the NOX inhibitor, GKT137831 (60 mg/kg per day; gavage) administered to diabetic and non-diabetic Apoe −/− mice for 10 weeks.
Results
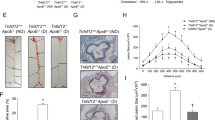
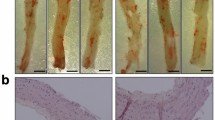
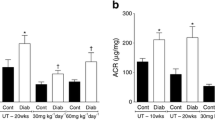
Diabetes increased atherosclerotic plaque development in the aortic sinus and this correlated with increased lesional accumulation of T cells and CD11c+ cells and altered T cell activation in the adjacent meLNs. Diabetic Apoe −/− mice demonstrated an elevation in vascular ROS production and expression of the proinflammatory markers monocyte chemoattractant protein 1, vascular adhesion molecule 1 and IFNγ. Blockade of NOX-derived ROS using GKT137831 prevented the diabetes-mediated increase in atherosclerotic plaque area and associated vascular T cell infiltration and also significantly reduced vascular ROS as well as markers of inflammation and plaque necrotic core area.
Conclusions/interpretation
Diabetes promotes pro-inflammatory immune responses in the aortic sinus and its associated lymphoid tissue. These changes are associated with increased ROS production by NOX. Blockade of NOX-derived ROS using the NOX inhibitor GKT137831 is associated with attenuation of these changes in the immune response and reduces the diabetes-accelerated development of atherosclerotic plaques in Apoe −/− mice.





Similar content being viewed by others
Abbreviations
- DC:
-
Dendritic cell
- DHE:
-
Dihydroethidium
- MCP-1:
-
Monocyte chemoattractant protein 1
- meLN:
-
Mediastinal lymph node
- NT:
-
Nitrotyrosine
- NOX:
-
NADPH oxidase
- ROS:
-
Reactive oxygen species
- VCAM-1:
-
Vascular adhesion molecule 1
References
Kannel WB, McGee DL (1979) Diabetes and cardiovascular disease. The Framingham study. J Am Med Assoc 241:2035–2038
Mäkinen VP, Forsblom C, Thorn LM et al (2009) Network of vascular diseases, death and biochemical characteristics in a set of 4,197 patients with type 1 diabetes (The FinnDiane Study). Cardiovasc Diabetol 8:54
Ross R (1999) Atherosclerosis - an inflammatory disease. N Engl J Med 340:115–126
Lassègue B, Griendling KK (2010) NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol 30:653–661
Hansson GK, Hermansson A (2011) The immune system in atherosclerosis. Nat Immunol 12:204–212
Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T (2007) Short-term high glucose exposure induces monocyte-endothelial cells adhesion and transmigration by increasing VCAM-1 and MCP-1 expression in human aortic endothelial cells. Atherosclerosis 193:328–334
Kanter JE, Bornfeldt KE (2013) Inflammation and diabetes-accelerated atherosclerosis: myeloid cell mediators. Trends Endocrinol Metab 24:137–144
Soro-Paavonen A, Watson AMD, Li J et al (2008) Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 57:2461–2469
Hansson GK, Holm J, Jonasson L (1989) Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol 135:169–175
Frostegård J, Ulfgren AK, Nyberg P et al (1999) Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145:33–43
Van Der Wal AC, Becker AE, van Der Loos CM, Das PK (1994) Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89:36–44
Baynes JW (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40:405–412
Griendling KK, Ushio-Fukai M (1997) NADH/NADPH oxidase and vascular function. Trends Cardiovasc Med 7:301–307
Hink U, Li H, Mollnau H et al (2001) Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88:E14–E22
Sedeek M, Callera G, Montezano A et al (2010) Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Ren Physiol 299:F1348–F1358
Guzik TJ, Sadowski J, Guzik B et al (2006) Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26:333–339
Ding H, Hashem M, Triggle C (2007) Increased oxidative stress in the streptozotocin-induced diabetic apoE-deficient mouse: changes in expression of NADPH oxidase subunits and eNOS. Eur J Pharmacol 561:121–128
Guzik TJ, Mussa S, Gastaldi D et al (2002) Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105:1656–1662
Wendt MC, Daiber A, Kleschyov AL et al (2005) Differential effects of diabetes on the expression of the gp91 phox homologues nox1 and nox4. Free Radic Biol Med 39:381–391
Gray SP, Di Marco E, Okabe J et al (2013) NADPH Oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127:1888–1902
Marumo T, Schini-Kerth VB, Fisslthaler B, Busse R (1997) Platelet-derived growth factor-stimulated superoxide anion production modulates activation of transcription factor NF-κB and expression of monocyte chemoattractant protein 1 in human aortic smooth muscle cells. Circulation 96:2361–2367
Kunsch C, Medford RM (1999) Oxidative stress as a regulator of gene expression in the vasculature. Circ Res 85:753–766
Cybulsky MI, Iiyama K, Li H et al (2001) A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Investig 107:1255–1262
Mach F, Sauty A, Iarossi AS et al (1999) Differential expression of three t lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Investig 104:1041–1050
Nareika A, Sundararaj KP, Im YB, Game BA, Lopes-Virella MF, Huang Y (2009) High glucose and interferon gamma synergistically stimulate MMP-1 expression in U937 macrophages by increasing transcription factor STAT1 activity. Atherosclerosis 202:363–371
Esposito K, Nappo F, Marfella R et al (2002) Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106:2067–2072
Hsueh W, Abel ED, Breslow JL et al (2007) Recipes for creating animal models of diabetic cardiovascular disease. Circ Res 100:1415–1427
Candido R, Jandeleit-Dahm KA, Cao Z et al (2002) Prevention of accelerated atherosclerosis by angiotensin-converting enzyme inhibition in diabetic apolipoprotein E-deficient mice. Circulation 106:246–253
Krege JH, Hodgin JB, Hagaman JR, Smithies O (1995) A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25:1111–1115
Aoyama T, Paik YH, Watanabe S et al (2012) Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56:2316–2327
Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA (1987) Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 68:231–240
Feng B, Zhang D, Kuriakose G, Devlin CM, Kockx M, Tabas I (2003) Niemann-Pick C heterozygosity confers resistance to lesional necrosis and macrophage apoptosis in murine atherosclerosis. Proc Natl Acad Sci U S A 100:10423–10428
Elmarakby AA, Loomis ED, Pollock JS, Pollock DM (2005) NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension 45:283–287
Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ (2011) Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 216:321–326
Kanter JE, Averill MM, Leboeuf RC, Bornfeldt KE (2008) Diabetes-accelerated atherosclerosis and inflammation. Circ Res 103:e116–e117
Stentz FB, Kitabchi AE (2005) Hyperglycemia-induced activation of human T-lymphocytes with de novo emergence of insulin receptors and generation of reactive oxygen species. Biochem Biophys Res Commun 335:491–495
Lindley S, Dayan CM, Bishop A, Roep BO, Peatman M, Tree TIM (2005) Defective suppressor function in CD4+CD25+ T cells from patients with type 1 diabetes. Diabetes 54:92–99
Kolbus D, Ramos OH, Berg KE et al (2010) CD8+T cell activation predominate early immune responses to hypercholesterolemia in Apoe−(/)− mice. BMC Immunol 11:58
Gupta S, Pablo AM, Jiang XC, Wang N, Tall AR, Schindler C (1997) IFN-γ, potentiates atherosclerosis in ApoE knock-out mice. J Clin Investig 99:2752–2761
Zhou X, Nicoletti A, Elhage R, Hansson GK (2000) Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation 102:2919–2922
Uyemura K, Demer LL, Castle SC et al (1996) Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Investig 97:2130–2138
McLaren JE, Ramji DP (2009) Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Rev 20:125–135
Kyaw T, Winship A, Tay C et al (2013) Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 127:1028–1039
Libby P, Ridker PM, Maseri A (2002) Inflammation and atherosclerosis. Circulation 105:1135–1143
Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92:657–671
Thim T, Hagensen MK, Bentzon JF, Falk E (2008) From vulnerable plaque to atherothrombosis. J Intern Med 263:506–516
Burke AP, Kolodgie FD, Zieske A et al (2004) Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol 24:1266–1271
Brito C, Naviliat M, Tiscornia AC et al (1999) Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol 162:3356–3366
Bobryshev YV (2010) Dendritic cells and their role in atherogenesis. Lab Investig 90:970–984
Sheng KC, Pietersz GA, Tang CK, Ramsland PA, Apostolopoulos V (2010) Reactive oxygen species level defines two functionally distinctive stages of inflammatory dendritic cell development from mouse bone marrow. J Immunol 184:2863–2872
Acknowledgements
The authors would like to thank E. Grixti for his expert assistance in measuring metabolic variables, M. Arnstein for assistance with general tissue pathology/immunohistochemistry and K. Gilbert for maintenance of the mice (all affiliated with Baker IDI Heart and Diabetes Institute, Melbourne, VIC, Australia). Appreciation extends to G. Paukovic (Burnet Institute, Melbourne, VIC, Australia) for his expertise in flow cytometry. Kind thanks are also given to A. Bobik (Department of Vascular Biology and Atherosclerosis, Baker IDI Heart and Diabetes Institute) for intellectual contribution to discussions.
Funding
This work was supported by the National Health and Medical Research Council project grant of Australia. KAJD is supported by an NHMRC senior Research Fellowship and MEC is an Australian Fellow for the NHMRC and a JDRF scholar. EDM is supported by a Post-graduate Fellowship (PB 12M 6943) from the National Heart Foundation of Australia.
Duality of interest
CS is a paid employee and owns shares in Genkyotex SA, Geneva, Switzerland. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Contribution statement
EDM contributed to the direction of the study, conducted data collection and subsequent analyses and wrote and edited the manuscript. SPG directed the study, conducted data analysis and wrote and edited the manuscript. PC performed and analysed some of the immunohistochemical stains and reviewed the manuscript. CK contributed to collection of meLNs, analysis of some immunohistochemistical stains and reviewed the manuscript. AZ assisted with flow cytometry data collection and analysis and reviewed the manuscript. CS was involved in the conception of the study design, contributed to the protocol for GKT137831 preparation and administration and reviewed and edited the manuscript. RMT and HHHWS assisted with data analysis of oxidative stress variables and also reviewed the manuscript. MEC contributed to data analysis and critical revisions of intellectual content of the manuscript. RS assisted with flow cytometry analysis and edited and reviewed the manuscript. KAJD contributed to the design and supervision of the study, provided intellectual input to discussions and edited and reviewed the manuscript. All authors approved the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
(PDF 50 kb)
ESM Table 2
(PDF 93 kb)
ESM Table 3
(PDF 73 kb)
ESM Table 4
(PDF 73 kb)
ESM Table 5
(PDF 74 kb)
ESM Fig. 1
(PDF 228 kb)
ESM Fig. 2
(PDF 99 kb)
ESM Fig. 3
(PDF 141 kb)
ESM Fig. 4
(PDF 113 kb)
Rights and permissions
About this article
Cite this article
Di Marco, E., Gray, S.P., Chew, P. et al. Pharmacological inhibition of NOX reduces atherosclerotic lesions, vascular ROS and immune–inflammatory responses in diabetic Apoe −/− mice. Diabetologia 57, 633–642 (2014). https://doi.org/10.1007/s00125-013-3118-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3118-3