Abstract
Aims/hypothesis
Type 2 diabetes and insulin resistance are often associated with the co-occurrence of coronary atherosclerosis and cardiac dysfunction. The aim of this study was to define the independent relationships between left ventricular dysfunction or ischaemia and patterns of myocardial perfusion and metabolism in type 2 diabetes.
Methods
Twenty-four type 2 diabetic patients—12 with coronary artery disease (CAD) and preserved left ventricular function and 12 with non-ischaemic heart failure (HF)—were enrolled in a cross-sectional study. Positron emission tomography (PET) was used to assess myocardial blood flow (MBF) at rest, after pharmacological stress and under euglycaemic hyperinsulinaemia. Insulin-mediated myocardial glucose disposal was determined with 2-deoxy-2-[18F]fluoroglucose PET.
Results
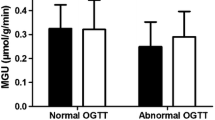
There was no difference in myocardial glucose uptake (MGU) between the healthy myocardium of CAD patients and the dysfunctional myocardium of HF patients. MGU was strongly influenced by levels of systemic insulin resistance in both groups (CAD, r = 0.85, p = 0.005; HF, r = 0.77, p = 0.01). In HF patients, there was an inverse association between MGU and the coronary flow reserve (r = −0.434, p = 0.0115). A similar relationship was observed in non-ischaemic segments of CAD patients. Hyperinsulinaemia increased MBF to a similar extent in the non-ischaemic myocardial of CAD and HF patients.
Conclusions/interpretation
In type 2 diabetes, similar metabolic and perfusion patterns can be detected in the non-ischaemic regions of CAD patients with normal cardiac function and in the dysfunctional non-ischaemic myocardium of HF patients. This suggests that insulin resistance, rather than diagnosis of ischaemia or left ventricular dysfunction, affects the metabolism and perfusion features of patients with type 2 diabetes.
Similar content being viewed by others
Introduction
Type 2 diabetes is associated with, and aggravates, the prognosis of ischaemic and non-ischaemic cardiomyopathy [1, 2]. The causal role of insulin resistance in non-ischaemic heart failure (HF) is well defined. Animal models have repeatedly demonstrated the existence of an insulin-resistant cardiomyopathy [3], which is characterised by an inefficient energy metabolism [3, 4] and can be reversed by improving energy (glucose) use [5]. In addition, clinical studies in humans strongly suggest a link between insulin resistance and non-ischaemic HF. Insulin resistance is highly prevalent in the non-ischaemic HF population [6]. It predates HF development [7], predicts the response to anti-adrenergic therapy [8] and independently defines a worse prognosis [9].
Despite such evidence, coronary artery disease (CAD) is considered the most common aetiology of HF in insulin-resistant diabetic patients [10]. It is thought that HF evolution in these patients results from a progressive loss of myocardial tissue due to the ischaemia, leading to the activation of several compensatory pathways which, in the long term, may become harmful for the heart [11]. Because diabetic patients are most commonly affected by a combination of left ventricular (LV) dysfunction and CAD, it is difficult to distinguish the independent relationships, if any, between myocardial insulin resistance and ischaemia or mechanical dysfunction/cardiomyopathy. Therefore, little is known about the potential role of myocardial dysmetabolism in the development of HF in diabetic CAD patients.
The myocardium normally responds to ischaemic injuries by altering its substrate metabolism to increase energy efficiency [12]. Insulin resistance prevents this adaptive response and can lead to further injury by contributing to lipotoxicity, sympathetic upregulation, inflammation, oxidative stress and fibrosis [13]. Although these pathways have been demonstrated in animal and in vitro studies, it is unknown whether, in humans, myocardial insulin resistance can affect cardiac metabolism in diabetic CAD patients before the development of HF (possibly contributing to the more rapid and more adverse evolution of CAD in these patients) or whether it is merely a consequence of the progressive reduction in LV function. This factor can have a crucial role in understanding mechanisms of disease evolution, influencing at the same time the treatment strategies to be undertaken in diabetic CAD patients at the time of diagnosis, i.e. before the development of contractile dysfunction.
In this study we selected well-controlled type 2 diabetic patients with non-ischaemic HF as a model of LV systolic dysfunction mediated by insulin resistance. We compared the metabolic and vascular features of their myocardial muscle with those of the non-ischaemic LV regions of well-matched type 2 diabetic patients with CAD in whom LV function was preserved. This study design allows investigation of whether early metabolic changes (similar to those recorded in non-ischaemic HF) are detectable in the non-ischaemic myocardium of patients with CAD and normal systolic LV function, which would suggest a central role of insulin resistance in the evolution of HF in individuals with diabetes and CAD.
Methods
Population
A total of 24 type 2 diabetic men were recruited: 12 had a diagnosis of CAD with normal LV systolic function and 12 had a diagnosis of non-ischaemic HF. Some data for the CAD patients have been previously reported [14]; here, we selected a very well-matched subgroup to target the comparison of CAD and HF. Patients in the non-ischaemic HF group were recruited in the context of a clinical trial (NCT00335465) from those with a diagnosis of dilated cardiomyopathy (DCM), according to current guidelines [15]; data collected during the baseline characterisation of the study were used for comparison with the CAD group. In both groups, significant co-morbid conditions (congenital, valvular, hypertensive, alcoholic heart disease, myocarditis and pericarditis as well as hepatic, endocrine—other than diabetes—or other major systemic diseases) were excluded using patients’ hospital records. Diagnosis of CAD was confirmed or excluded by coronary angiography and stress single-photon emission computed tomography (SPECT), while type 2 diabetes was diagnosed according to the 2003 criteria of the ADA [16]. Patients with atrial fibrillation were excluded because of the difficulty in obtaining reliable estimates of LV ejection fraction and because of the potential independent effects of arrhythmia on myocardial blood flow. Furthermore, significant co-morbid conditions were excluded using patients’ hospital records. The study was approved by the local ethical committees and all individuals provided a written informed consent before taking part in study procedures.
Baseline characterisation
LV structure and function were determined by transthoracic two-dimensional echocardiography and LV ejection fraction was calculated from apical four-chamber and two-chamber views by the biplane summation method [17]. Blood samples were collected to assess the metabolic and cardiovascular risk profile. Electrocardiogram, systolic and diastolic blood pressure (BP), heart rate, body weight and height of each patient were recorded in resting conditions. Baseline systemic vascular resistances (SVRs) were calculated as follows: \( {\text{SVR}} = {\text{MABP}}/\left( {{\text{SV}} \times {\text{HR}}} \right) \), where MABP is the mean arterial BP, SV is stroke volume and HR is heart rate [18].
Positron emission tomography studies
All patients were studied in the absence of morning medical therapy and after an overnight fasting period; caffeine, theophylline and theophylline derivatives were withdrawn 24 h before imaging. Positron emission tomography (PET) examinations were performed in two different sessions. During the first session (perfusion study), resting and stress (adenosine) blood flow in the myocardium were evaluated. Adenosine-stimulated coronary flow reserve (CFR) was defined as the ratio of adenosine-stimulated to resting blood flow values. During the second session (metabolic study), insulin-mediated blood flow and glucose consumption in the myocardium were evaluated during a hyperinsulinaemic–euglycaemic clamp. To assess the effect of insulin infusion on coronary blood flow the ratio between coronary blood flow values recorded during the euglycaemic clamp and those recorded at rest during the perfusion study was calculated (insulin-stimulated coronary blood flow [iCBF]).
Perfusion study
In patients with non-ischaemic HF, myocardial blood flow was evaluated with the use of [13N]ammonia; [15O]water was used in the CAD group. The procedure for blood flow measurements at rest and during pharmacologically induced stress has been previously described [14]. Open-access software was used to analyse the final images (Carimas 2.0, Turku, Finland). The reconstructed transaxial images were reoriented into short-axis views of the left ventricle. For each patient, values of myocardial blood flow (MBF) were computed in the whole left ventricle as well as in three different LV regions, corresponding to the vascular beds of the three main coronary arteries. Ischaemic or non-ischaemic vascular beds were defined according to results obtained during stress single-photon emission tomography, further confirmed by coronary angiography. To allow comparison of the relative changes in coronary blood flow between resting and stimulated states, MBF values obtained with both [13N]ammonia and [15O]water were referred to the average myocardial perfusion in each region of interest (either the whole LV myocardium or the myocardium subtending each vascular bed). Therefore, in CAD patients the final values of MBF obtained with [15O]water were not normalised to the perfusable tissue fraction (normalisation that is not possible for [13N]ammonia and leads to greater values of blood flow).
Metabolic study
Patients were positioned on the bed of the tomograph and a baseline blood sample was obtained for the measurement of glucose, insulin and NEFA. Serum insulin was then acutely raised using a primed-continuous (1 mU kg−1 min−1) infusion of regular human insulin. Normoglycaemia was based on plasma glucose measurements performed every 5 min on arterialised blood, and whole-body insulin-stimulated glucose uptake (M value) was calculated from the glucose infusion rate during the period of PET scanning, and expressed per kg body weight (μmol kg−1 min−1), according to the clamp technique, as previously described [19]. Plasma samples were collected every 30 min for the determination of NEFA and insulin levels. In the first hour of the clamp, a bolus of [13N]ammonia or [15O]water was injected, and a dynamic PET scan was performed, according to the perfusion study protocol, to evaluate blood flow in the myocardium. After decay of the residual tracer, a 2-deoxy-2-[18F]fluorodeoxyglucose ([18F]FDG) bolus of ∼185 MBq was administered over <1 min, and a dynamic scan was started immediately to evaluate insulin-stimulated glucose uptake in myocardium.
[18F]FDG image analysis was performed using the Carimas software. Myocardial glucose fractional uptake rates were estimated using Patlak graphical analysis [20], and multiplied by plasma glucose levels to obtain myocardial glucose uptake (MGU). A lumped constant of 1.0 was assumed [21]. The same LV segmentation reported in the perfusion study was used to compute the glucose uptake in the whole left ventricle as well as in each coronary vascular bed.
Blood samples
Plasma glucose, total serum cholesterol, triacylglycerols and HDL-cholesterol levels were assessed using standard procedures while LDL-cholesterol levels were calculated according to the Friedewald formula [22]. Glycosylated haemoglobin was assayed by high performance liquid chromatography. Insulin levels were measured by immunoenzymatic assay, and plasma glucose was measured by the glucose oxidase reaction (Beckman Glucose Analyser, Fullerton, CA, USA). NEFA were determined spectrophotometrically (NEFA-C kit, Wako Chemicals, Neuss, Germany).
Statistical analysis
Results were analysed using SPSS (version 17) statistical software package. Data are presented as mean ± SD. Analysis of variance or unpaired and paired t tests were used to identify significant between-group or within-group differences, respectively. Regression analyses were performed by standard methods. As two different tracers were used to measure myocardial perfusion, comparisons were only made within the same group and between the relative changes induced by adenosine and insulin over baseline MBF. The average value of MGU in non-ischaemic vascular beds of the CAD group was used in the comparison with the non-ischaemic myocardium of the HF group. A p value ≤0.05 was considered to be statistically significant.
Results
Table 1 reports the baseline characteristics in the study groups. The two groups were fully matched for age, sex and metabolic and cardiovascular risk factors. As per study design, cardiac variables were significantly different, with individuals in the CAD group displaying normal LV systolic function and end-diastolic diameters. During the euglycaemic insulin clamp, serum NEFA concentrations decreased significantly (p < 0.001) from baseline as expected. Plasma glucose was normalised and maintained within euglycaemic levels similarly in the two groups.
Adenosine infusion significantly increased resting MBF in both groups (p < 0.001 for both; CFR in CAD: 1.65 ± 0.27; CFR in HF: 1.80 ± 0.18). Similarly, insulin infusion during the clamp significantly increased MBF from baseline levels in the CAD group (p < 0.001, iCBF = 1.40 ± 0.48) as well as in the HF group (p < 0.01, iCBF = 1.26 ± 0.52). The increase in coronary flow recorded during the clamp did not differ between non-ischaemic regions of the CAD group and the myocardium of the HF group (Fig. 1), whereas it tended to be higher in ischaemic than non-ischaemic vascular beds (p = 0.04). The only variable predicting the overall increase in blood flow during the clamp was the value of peripheral vascular resistance (r = −0.47, p = 0.027). The stimulation of MBF by hyperinsulinaemia was not associated with any change in the rate–pressure product between the two PET sessions.
MGU, insulin-stimulated blood flow (iCBF) and adenosine-stimulated coronary blood flow (CFR, coronary flow reserve) in groups with non-ischaemic HF (white bars) and non-ischaemic vascular beds of CAD (black bars). Data are presented as mean ± SD. There were no statistically significant differences between the two groups
There was no difference in MGU between non-ischaemic vascular beds of the CAD group and the myocardium of the HF group (Fig. 1).
A strong and positive association was found between MGU and whole-body insulin-stimulated glucose uptake (r = 0.71, p < 0.001; Fig. 2). A similar association was also detected within each group (CAD, r = 0.85, p = 0.005; HF, r = 0.77, p = 0.01), as well as in ischaemic and non-ischaemic vascular beds alone (ischaemic, r = 0.81, p = 0.007; non-ischaemic, r = 0.79, p < 0.001). In the HF group, MGU was negatively associated with values of CFR (r = −0.434, p = 0.0115). A similar relationship was found in non-ischaemic vascular beds of the CAD group (r = −0.538, p = 0.026), but not in the ischaemic segments. Instead, across ischaemic but not non-ischaemic segments, there was a strong and negative association between the increase in myocardial blood flow induced by insulin and MGU (r = −0.670, p = 0.002).
The similar patterns of myocardial perfusion and metabolism detected in non-ischaemic regions of CAD and HF patients were unlikely to be due to pharmacological treatments, as Table 1 shows that patient medications were significantly different between the two groups (Table 1).
As the degree of NEFA suppression during clamp differed between groups and NEFA levels are known potentially to influence MGU and MBF, we adjusted our statistical analyses for baseline and clamp-induced NEFA concentrations, as well as for the magnitude of NEFA suppression obtained with insulin infusion; results were not materially affected.
Discussion
To the best of our knowledge, there are no previous studies in which patterns of myocardial metabolism and perfusion in type 2 diabetic patients with non-ischaemic HF has been compared with that of type 2 diabetic patients with CAD and normal systolic LV function. Notably, the two groups were carefully matched for all metabolic and cardiovascular risk factors so as to leave only their cardiac disease to account for any differences. In addition, the absence of HF in CAD patients and vice versa eliminated the confounding overlap of coronary obstruction and mechanical cardiac dysfunction usually seen in diabetic patients with heart disease. Our key finding is that similar characteristics of MGU and perfusion can be detected in the non-ischaemic vascular beds of patients with CAD but normal LV function and those of patients with non-ischaemic HF. This is primarily due to the strong influence of insulin resistance on myocardial metabolism observed in both groups. Indeed, we found that levels of insulin resistance are likely to set the magnitude of LV MGU, though the relative distribution of MGU among different LV regions seems to be influenced by the local values of coronary flow reserve. Additionally, euglycaemic hyperinsulinaemia significantly raises resting myocardial blood flow in non-ischaemic HF patients as well as in CAD patients with normal LV function. This effect was observed in both ischaemic and non-ischaemic LV regions. Interestingly, the magnitude of the insulin-mediated increase in blood flow was similar in non-ischaemic segments of CAD and HF groups.
There is epidemiological evidence that insulin resistance precedes HF rather than occurring as its consequence [7, 23]. The pathophysiological mechanism commonly advocated to explain this finding assumes that reduced myocardial sensitivity to the insulin signal would prevent the shift from NEFA to glucose consumption that is necessary to preserve contractile efficiency under stress or ischaemic conditions [13]. Current data strongly substantiate this hypothesis by revealing a strong dependency of MGU on insulin resistance. We further observed that, in healthy myocardial regions of patients with CAD, the distribution of LV metabolism is influenced by the CFR. This suggests that blood flow remains an important determinant of the regional MGU, independent of the global levels of myocardial insulin resistance.
Previous evidence demonstrated that the prognostic impact of insulin resistance in HF patients is independent of values of LV ejection fraction [9]. Our results confirm this epidemiological finding and provide possible mechanistic explanations. Indeed, the occurrence of similar metabolic and vascular characteristics in non-ischaemic beds of CAD and HF patients suggests that in type 2 diabetes the impact of insulin resistance on cardiac metabolism/perfusion is independent of LV function. Moreover, in HF patients the reduced values of CFR and the relative increase in MGU observed in regions with a more pronounced reduction in CFR suggest that a metabolic response typical of the ischaemic myocardium occurs also in patients without coronary disease. In this specific situation, insulin resistance might play a crucial role in the evolution of LV systolic dysfunction, by opposing the metabolic adaptation of the failing myocardium, further reducing its contractile efficiency.
The ability of an acute insulin infusion to induce an increase in myocardial blood flow has been previously reported in healthy individuals as well as in type 2 diabetic patients with and without CAD [14, 24, 25]. We now describe a similar effect in patients with type 2 diabetes and non-ischaemic HF. In humans, the mechanisms accounting for the vasodilatory actions of insulin have been primarily studied in peripheral vessels and comprise a spatially and temporally heterogeneous process that occurs in distinct stages [26]. First, dilation of terminal arterioles increases the number of perfused capillaries (capillary recruitment) within a few minutes without concomitant changes in total blood flow. At high doses of insulin infusion this is followed by relaxation of larger resistance vessels, which increases overall local blood flow [27]. While we cannot provide direct evidence for these mechanisms, our current data support the possibility that similar pathways are operating in the hearts of diabetic patients. Indeed, peripheral vascular resistance was the only predictor of MBF response to insulin infusion.
The current study has strengths and limitations. The use of PET allows in vivo investigation of regional cardiac metabolism, avoiding the assumption that systemic insulin resistance extends to the myocardium. The direct measurement of the interaction between systemic and myocardial metabolism provides quantitative proof and estimation of the above concept and a mechanistic explanation of previous epidemiological findings. Furthermore, the use of gold-standard techniques to assess myocardial and whole-body insulin resistance increases the robustness of our results and conclusions. Finally, the selection of patients with non-ischaemic HF allows the independent relationship between LV systolic function and cardiac perfusion/metabolism to be studied in patients with type 2 diabetes, removing the confounding effect of CAD. Likewise, the selection of patients with CAD and normal LV function allows the independent impact of coronary obstruction to be addressed, without the additive effect of a disturbance in the mechanical cardiac performance.
The lack of longitudinal data represents the main limitation of the current report and prevents us from making definitive conclusions regarding the impact of metabolic patterns on HF evolution. Because different tracers were used to study myocardial perfusion, the inter-group analyses had to be restricted to the comparable variables (MGU and adenosine- and insulin-mediated changes in flow). Instead, absolute values of cardiac perfusion could be used in testing the correlations between blood flow and metabolism within the same patient. The estimation of MGU by PET does not provide information related on the intracellular pathways of glucose use. In CAD, the accumulation of intracellular lactate contributes to reduce contractile function in ischaemic vascular beds [28], while similar mechanisms remain unexplored in non-ischaemic HF. Therefore, our results cannot exclude that different intracellular metabolic processes may underlie similar patterns of MGU.
Conclusion
In patients with type 2 diabetes, metabolic patterns of subclinical ischaemia and insulin resistance can be detected in individuals with CAD and normal ventricular function as well as in those with non-ischaemic HF. This suggests that in type 2 diabetic patients MGU is mostly under the control of insulin-mediated signals, whereas the presence of LV systolic dysfunction or coronary obstruction only marginally affects the regional distribution of myocardial metabolism. Therapies aimed at improving myocardial insulin sensitivity (e.g. pioglitazone) offer the most rational approach for type 2 diabetic patients. In a manner similar to that in healthy and CAD individuals with type 2 diabetes, insulin increases coronary blood flow in patients with non-ischaemic HF. Further studies are needed to examine if the beneficial effects of insulin on the myocardium extend from an acute infusion to a chronic regimen, potentially representing a treatment choice in patients with HF.
Abbreviations
- CAD:
-
Coronary artery disease
- CFR:
-
Coronary flow reserve
- [18F]FDG:
-
2-Deoxy-2-[18F]fluorodeoxyglucose
- HF:
-
Heart failure
- iCBF:
-
Insulin-stimulated coronary blood flow
- LV:
-
Left ventricular
- M :
-
Whole-body insulin-stimulated glucose uptake
- MBF:
-
Myocardial blood flow
- MGU:
-
Myocardial glucose uptake
- PET:
-
Positron emission tomography
References
From AM, Leibson CL, Bursi F et al (2006) Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med 119:591–599
Stamler J, Vaccaro O, Neaton JD, Wentworth D (1993) Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 16:434–444
Belke DD, Larsen TS, Gibbs EM, Severson DL (2000) Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab 279:E1104–E1113
Finck BN, Han X, Courtois M et al (2003) A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci USA 100:1226–1231
Semeniuk LM, Kryski AJ, Severson DL (2002) Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol 283:H976–H982
Witteles RM, Tang WH, Jamali AH, Chu JW, Reaven GM, Fowler MB (2004) Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J Am Coll Cardiol 44:78–81
Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L (2005) Insulin resistance and risk of congestive heart failure. JAMA 294:334–341
O’Keefe JH Jr, Magalski A, Stevens TL et al (2000) Predictors of improvement in left ventricular ejection fraction with carvedilol for congestive heart failure. J Nucl Cardiol 7:3–7
Paolisso G, Tagliamonte MR, Rizzo MR et al (1999) Prognostic importance of insulin-mediated glucose uptake in aged patients with congestive heart failure secondary to mitral and/or aortic valve disease. Am J Cardiol 83:1338–1344
MacDonald MR, Petrie MC, Hawkins NM et al (2008) Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur Heart J 29:1224–1240
Boudina S, Abel ED (2007) Diabetic cardiomyopathy revisited. Circulation 115:3213–3223
Taegtmeyer H, McNulty P, Young ME (2002) Adaptation and maladaptation of the heart in diabetes: part I: general concepts. Circulation 105:1727–1733
Young ME, McNulty P, Taegtmeyer H (2002) Adaptation and maladaptation of the heart in diabetes: part II: potential mechanisms. Circulation 105:1861–1870
Lautamaki R, Airaksinen KE, Seppanen M et al (2006) Insulin improves myocardial blood flow in patients with type 2 diabetes and coronary artery disease. Diabetes 55:511–516
Elliott P, Andersson B, Arbustini E et al (2008) Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 29:270–276
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (2003) Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 26(Suppl 1):S5–S20
Schiller NB, Acquatella H, Ports TA et al (1979) Left ventricular volume from paired biplane two-dimensional echocardiography. Circulation 60:547–555
Tuunanen H, Engblom E, Naum A et al (2008) Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation 118:1250–1258
DeFronzo RA, Tobin JD, Andres R (1979) Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223
Patlak CS, Blasberg RG (1985) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5:584–590
Bøtker HE, Böttcher M, Schmitz O et al (1997) Glucose uptake and lumped constant variability in normal human hearts determined with [18F]fluorodeoxyglucose. J Nucl Cardiol 4:125–132
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Arnlov J, Lind L, Zethelius B et al (2001) Several factors associated with the insulin resistance syndrome are predictors of left ventricular systolic dysfunction in a male population after 20 years of follow-up. Am Heart J 142:720–724
Iozzo P, Chareonthaitawee P, Di TM, Betteridge DJ, Ferrannini E, Camici PG (2002) Regional myocardial blood flow and glucose utilization during fasting and physiological hyperinsulinemia in humans. Am J Physiol Endocrinol Metab 282:E1163–E1171
Iozzo P, Chareonthaitawee P, Rimoldi O, Betteridge DJ, Camici PG, Ferrannini E (2002) Mismatch between insulin-mediated glucose uptake and blood flow in the heart of patients with type II diabetes. Diabetologia 45:1404–1409
Vincent MA, Dawson D, Clark AD et al (2002) Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 51:42–48
Baron AD, Tarshoby M, Hook G et al (2000) Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes 49:768–774
Stanley WC (2001) Cardiac energetics during ischaemia and the rationale for metabolic interventions. Coron Artery Dis 12(Suppl 1):S3–S7
Acknowledgements
We are grateful to the staff of the PET Centre at the Institute of Clinical Physiology (Pisa, Italy) and of the Turku PET Centre (Turku, Finland) for their excellent technical assistance.
Funding
This study was funded by sanofi-aventis as part of a clinical trial (NCT00335465), the Aarne and Aili Turunen Foundation, the Finnish Cultural Foundation, the Jalmari and Rauha Ahokas Foundation and GlaxoSmithKline.
Duality of interest
The authors confirm that there is no duality of interest associated with this paper.
Contribution statement
PI, EF, DN, PN, JK and PAS designed the study. SM, RL, LG, PDC, CP, SP, VC, AMS, ME, MAM and DN collected data. PI, SM, RL, LG and PN analysed and interpreted the data. SM, PI and RL prepared the manuscript. SM, PI, EF, DN, ME, MAM, PN, JK, PAS, RL, LG, PDC, CP, SP, VC and AMS critically revised the manuscript. All authors have approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masi, S., Lautamäki, R., Guiducci, L. et al. Similar patterns of myocardial metabolism and perfusion in patients with type 2 diabetes and heart disease of ischaemic and non-ischaemic origin. Diabetologia 55, 2494–2500 (2012). https://doi.org/10.1007/s00125-012-2631-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2631-0