Abstract
Aims/hypothesis
Sirtuin-1 (SIRT1) is a potential therapeutic target to combat insulin resistance and type 2 diabetes. This study aims to identify a microRNA (miRNA) targeting SIRT1 to regulate hepatic insulin sensitivity.
Methods
Luciferase assay combined with mutation and immunoblotting was used to screen and verify the bioinformatically predicted miRNAs. miRNA and mRNA levels were measured by real-time PCR. Insulin signalling was detected by immunoblotting and glycogen synthesis. Involvement of SIRT1 was studied with adenovirus, inhibitor and SIRT1-deficient hepatocytes. The role of miR-181a in vivo was explored with adenovirus and locked nucleic acid antisense oligonucleotides.
Results
miR-181a targets the 3′ untranslated region (3′UTR) of Sirt1 mRNA through a miR-181a binding site, and downregulates SIRT1 protein abundance at the translational level. miR-181a is increased in insulin-resistant cultured hepatocytes and liver, and in the serum of diabetic patients. Overexpression of miR-181a decreases SIRT1 protein levels and activity, and causes insulin resistance in hepatic cells. Inhibition of miR-181a by antisense oligonucleotides increases SIRT1 protein levels and activity, and improves insulin sensitivity in hepatocytes. Ectopic expression of SIRT1 abrogates the effect of miR-181a on insulin sensitivity, and inhibition of SIRT1 activity or SIRT1 deficiency markedly attenuated the improvement in insulin sensitivity induced by antisense miR-181a. In addition, overexpression of miR-181a by adenovirus impairs hepatic insulin signalling, and intraperitoneal injection of locked nucleic acid antisense oligonucleotides for miR-181a improves glucose homeostasis in diet-induced obesity mice.
Conclusions/interpretation
miR-181a regulates SIRT1 and improves hepatic insulin sensitivity. Inhibition of miR-181a might be a potential new strategy for treating insulin resistance and type 2 diabetes.
Similar content being viewed by others
Introduction
In recent years, type 2 diabetes mellitus has become a common chronic disease worldwide, a critical factor contributing to the pathogenesis of type 2 diabetes being insulin resistance [1, 2]. Sirtuin-1 (SIRT1), an NAD-dependent protein deacetylase, has been shown as a potential therapeutic target to combat insulin resistance and type 2 diabetes [3–5].
It has been reported that SIRT1 is a positive regulator of insulin signalling in skeletal muscle cells, adipocytes and hepatocytes [6–8]. Adenovirus-mediated overexpression of SIRT1 in the liver of insulin-resistant mice attenuates hepatic steatosis and ameliorates systemic insulin resistance [9]. Moderate transgenic overexpression of Sirt1 in db/db and high-fat diet-fed mice improves glucose tolerance and insulin sensitivity [10, 11]. Aged mice orally administered resveratrol, which can enhance SIRT1 activity, display increased insulin sensitivity and survival [12]. Resveratrol and its structural analogues, especially SRT1720, also protect mice against high-fat diet-induced obesity (DIO) and insulin resistance [13–15]. Further studies focused on the regulators of SIRT1 might provide potential new therapeutic approaches to combat insulin resistance and type 2 diabetes.
MicroRNAs (miRNAs) have been associated with many diseases, including type 2 diabetes [16, 17]. miR-278 mediates energy balance by regulating insulin responsiveness in Drosophila, and miR-278 mutants are insulin resistant [18]. Aberrant expression of miRNAs has been observed in the insulin-target tissues of rat and mouse models of type 2 diabetes [19–21]. In adipocytes, miR-320 induces insulin resistance by inhibiting the insulin–phosphoinositide 3-kinase (PI3K) signalling pathway [22]. miR-29, which has been found to be elevated in the skeletal muscle of diabetic rats and the liver of db/db mice, negatively regulates insulin signalling via inhibiting the p85α subunit of PI3K [23, 24]. In muscle cells, miR-7 downregulates IRS-1 levels as well as inhibiting insulin-stimulated Akt phosphorylation and glucose uptake [25]. However, the role of miRNAs in regulating hepatic insulin sensitivity is still largely unknown.
In the present work, we sought to identify a miRNA targeting Sirt1 to regulate hepatic insulin sensitivity in vitro and in vivo, and to provide a potential approach for combating hepatic insulin resistance and type 2 diabetes.
Methods
Plasmid construction
Plasmid construction was performed as described elsewhere (electronic supplementary material [ESM] Methods). The primer sequences are shown in ESM Table 1.
Bioinformatics analysis
Human, mouse and rat Sirt1 3′ untranslated region (3′UTR) sequences were retrieved from the Entrez Nucleotide database (http://www.ncbi.nlm.nih.gov/nuccore). The potential miRNA targets within the conserved regions in 3′UTR of Sirt1 were predicted by miRBase (www.mirbase.org/), TargetScan (www.targetscan.org/), PicTar (http://pictar.mdc-berlin.de/) and miRanda (www.microrna.org/microrna/home.do). Sirt1 3′UTR sequences from human, chimpanzee, dog, rat, mouse, chicken and pufferfish were aligned with each other and with the miR-181a seed region using PicTar.
Cell culture and treatments
HEK293T and HepG2 cells were maintained in DMEM with 10% FBS. Primary cultured mouse hepatocytes were prepared from 9-week-old C57BL/6 mice or the mice with the indicated genotypes by the collagenase perfusion method as described previously [26], and cultured in DMEM with 10% FBS.
For transfection, HEK293T, HepG2 cells or primary cultured mouse hepatocytes in 12-well plates were transfected with the indicated plasmids (1.6 μg/well), miRNA mimics, antisense oligonucleotides or small interfering RNA (siRNA) at the indicated final concentrations in the culture medium using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After transfection for 72 h, cells were subsequently stimulated with or without 100 nmol/l insulin (Sigma, St Louis, MO, USA) for 15 min and harvested for immunoblotting. To induce insulin resistance, HepG2 cells or primary cultured mouse hepatocytes were transfected for 54 h and then treated with 18 mmol/l glucosamine for 18 h in DMEM with 5 mmol/l glucose, before being stimulated with or without insulin for immunoblotting or measurement of glycogen synthase activity and glycogen synthesis assay. For adenovirus infection, HepG2 cells were infected with the indicated adenovirus at a multiplicity of infection of 50 for 8 h before transfection. To inhibit the activity of SIRT1, HepG2 cells were treated with 10 mmol/l nicotinamide or 50 μmol/l Sirtinol (Sigma) for an additional 12 h after transfection with antisense miR-181a at the indicated concentrations for 60 h. For the experiment considering insulin-induced insulin resistance, HepG2 cells were treated with 100 nmol/l insulin for 24 h. To upregulate SIRT1 protein level, HepG2 cells were treated with 0.5 mmol/l AICAR (Sigma) for 48 h.
C2C12 myoblasts were maintained in DMEM with 10% FBS and differentiated in DMEM with 2% horse serum after reaching confluence. After 4 days, the C2C12 cells were differentiated into myotubes and transfected with miR-181a mimics for 72 h at the indicated concentrations.
RNA isolation and real-time PCR
Total RNA was prepared using TRIzol reagent (Invitrogen). To measure mRNA levels, total RNA was reverse-transcribed and quantified by real-time PCR using Power SYBR Green (Applied Biosystems, Foster City, CA, USA). The primer sequences are shown in ESM Table 1. miR-181a was reverse-transcribed using a miR-181a-specific stem-loop primer (Applied Biosystems), and subsequently measured by real-time PCR using miR-181a-specific Taqman probes (Applied Biosystems). U6 (also known as Rnu6) was similarly measured and used for normalisation of miR-181a expression.
miRNA mimics, miRNA antisense oligonucleotides, siRNA and locked nucleic acid antisense oligonucleotides
miRNA-181a duplex mimics and 2′-O-methylated single-stranded miR-181a antisense oligonucleotides (anti-181a) were obtained from GenePharma (Shanghai, China). Irrelevant miRNA duplex mimics for Caenorhabditis elegans cel-miR-239b or 2′-O-methylated single-stranded cel-miR-239b antisense oligonucleotides were co-transfected as a mock control to equalise the total amount of miRNA or antisense oligonucleotides, respectively, for each transfection as described previously [27]. SIRT1 and its control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Locked nucleic acid (LNA) antisense oligonucleotides specific for miR-181a (LNA-anti-181a) and mismatched LNA oligonucleotides (LNA-control) were obtained from Exiqon (Vedbaek, Denmark).
Luciferase assay
HEK293T cells in a 24-well plate were co-transfected with pSIF-GFP or the indicated plasmids expressing miRNA precursors (0.8 μg/well), pRL-Sirt1-3′UTR (pRL-TK vector containing Sirt1 3′UTR) or pRL-Sirt1-3′UTRm (pRL-TK vector containing mutant Sirt1 3′UTR) (0.1 μg/well) and pSV40-β-gal (Promega, Madison, WI, USA) (0.1 μg/well) using Lipofectamine 2000. HepG2 cells in a 24-well plate were co-transfected with the indicated miRNA mimics, pRL-Sirt1-3′UTR (0.1 μg/well) and pSV40-β-gal (0.1 μg/well) using Lipofectamine 2000. After transfection for 72 h, cells were harvested for luciferase assay as previously described [6].
Immunoblotting
Immunoblotting was performed with antibodies against SIRT1 (Millipore, Billerica, MA, USA), α-tubulin (Sigma), GAPDH (Millipore), insulin receptor (INSR), Tyr1150/1151-phosphorylated INSR, Akt, Ser473-phosphorylated Akt, glycogen synthase kinase-3β (GSK3β), Ser9-phosphorylated GSK3β, SH2 domain-containing protein tyrosine phosphatase 2 (SHP2), acetyl-p53(K382), p53, acetyl-histone H3(K9), histone H3 (Cell Signaling, Beverly, MA, USA), G6Pase (Santa Cruz Biotechnology) and phosphoenolpyruvate carboxykinase (Novus, Littleton, CO, USA). Protein quantification was analysed by Quantity One software (Bio-Rad, Hercules, CA, USA), and normalised to α-tubulin or GAPDH.
Measurement of SIRT1 activity
SIRT1 deacetylase activity was determined using a SIRT1 Fluorimetric Activity Assay Kit (Biomol International, New York, NY, USA). Nuclear extracts from HepG2 cells were used for measuring SIRT1 deacetylase activity. Liver samples from mice fed chow or high-fat diet were lysed by radioimmunoprecipitation assay buffer, and then SIRT1 was immunoprecipitated to detect its deacetylase activity.
NAD assay
Details of the NAD assay are shown in the ESM Methods.
Human study
The fasting plasma of age-matched male control and diabetic participants was collected at the Centre Hospital of Xuhui District, Shanghai. Patients with fasting plasma glucose >7.0 mmol/l were considered to have diabetes, as previously described [28]. Written informed consent was obtained from each participant, and the study was approved by the Institutional Review Board of the Institute for Nutritional Sciences. Serum RNA was isolated using TRIzol LS reagent (Invitrogen).
Measurement of glycogen synthase activity and glycogen synthesis assay
Glycogen synthase activity was determined using a modified method as previously described [6]. HepG2 cells were treated with or without 100 nmol/l insulin for 10 min and harvested for glycogen synthase activity assay using uridine diphosphate-[3H]glucose (GE Healthcare, Piscataway, NJ, USA). Glycogen synthesis was determined as previously described [6]. After transfection with the indicated miRNA mimics or antisense oligonucleotides at the indicated final concentrations for 72 h, HepG2 cells were processed with or without 100 nmol/l insulin and 3.7 × 104 Bq/ml [3H]glucose (Amersham, Van Nuys, CA, USA) in DMEM with 5 mmol/l glucose for 3 h, and then harvested for glycogen synthesis assay.
Generation of recombinant adenoviruses
To construct adenoviruses for overexpression of GFP, human SIRT1 or mouse miR-181a, complementary DNAs encoding mouse pre-miR-181a, human SIRT1 and GFP were inserted into pShuttle-CMV vector (Stratagene), and then subcloned into pAdEasy-1 adenoviral backbone vector (Stratagene). Recombinant adenoviruses were then generated following the manufacturer's protocol. Briefly, the adenoviral constructs were linearised with the restriction enzyme PacI and transfected into HEK293A cells using Lipofectamine 2000. After several rounds of propagation, the recombinant adenoviruses were purified by CsCl gradient centrifugation.
Animal experiments
Details of the animal experiments are given in the ESM Methods.
Statistics
Data are expressed as means±SD of at least three independent experiments. Statistical significance was assessed by Student’s t test except where indicated. Relationships between variables were determined by the Pearson correlation coefficient test. Differences were considered statistically significant at p < 0.05.
Results
miR-181a targets Sirt1 3′UTR through an miR-181a binding site
To identify the miRNAs targeting Sirt1 3′UTR, the miRNA target-prediction programs miRBase, miRanda, PicTar and TargetScan were used to obtain the potential miRNAs targeting Sirt1 3′UTR. Luciferase reporter under the control of Sirt1 3′UTR was used to examine the 18 selected potential miRNAs. As shown in Fig. 1a, overexpression of miR-181a, miR-543, miR-30a, miR-199b or miR-200a significantly downregulated the activity of the Sirt1 3′UTR, and miR-181a showed the most significant effect of all the miRNAs tested. Furthermore, we found that miR-181a, miR-543 and miR-200a could significantly reduce the luciferase activity of Sirt1 3′UTR in HepG2 cells as well as in HEK293T cells (ESM Fig. 1).
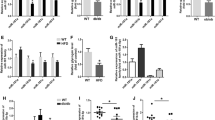
MiR-181a targets Sirt11 3′UTR. a Among the 18 predicted miRNAs, miR-181a showed the most significant repressive effect on the activity of Sirt1 3′UTR. After transfection with pRL-Sirt1-3′UTR and the indicated plasmids for 72 h, HEK293T cells were harvested for luciferase assay. *p < 0.05, **p < 0.01 vs pSIF-GFP (pSIF). b Sequence alignment of human (Hsa) miR-181a with the 3′UTRs of chimpanzee (Ptr), dog (Cfa), rat (Rno), mouse (Mmu), chicken (Gga) and pufferfish (Fru) Sirt1. The seed region of miR-181a is indicated in bold. c Mutation of the predicted miR-181a binding site in Sirt1 3′UTR abrogated the repressive effect of miR-181a on the activity of Sirt1 3′UTR when measured by luciferase assay. Black bars, pSIF; grey bars, pSIF-181a. **p < 0.01 vs pSIF or mutant reporter. d Mutation of the miR-181a seed region abrogated the repressive effect of miR-181a on the activity of Sirt1 3′UTR. **p < 0.01 vs pSIF or pSIF-181a-m. Error bars represent SD
To explore whether miR-181a directly targets Sirt1 3′UTR, we analysed the Sirt1 3′UTRs in human, chimpanzee, dog, rat, mouse, chicken and pufferfish by PicTar, and found that the potential binding site for miR-181a within Sirt1 3′UTRs is highly conserved from chicken to human (Fig. 1b). To test whether miR-181a represses the activity of Sirt1 3′UTR through this conserved site, we generated a reporter with a deletion of this site (Fig. 1c, upper panel). As expected, overexpression of miR-181a had no effect on the mutant reporter (Fig. 1c, bottom panel). To further confirm whether miR-181 targets Sirt1 3′UTR, a plasmid expressing mutant miR-181a with two mismatched bases in the seed region was constructed (Fig. 1d, upper panel). We found that the mutant miR-181a had no effect on the activity of Sirt1 3′UTR (Fig. 1d, bottom panel). These data demonstrate that miR-181a targets Sirt1 3′UTR through its binding site within Sirt1 3′UTR.
miR-181a decreases SIRT1 protein abundance at the translational level
To examine whether miR-181a downregulates SIRT1 protein level, immunoblotting was performed with HEK293T cells transfected with the indicated plasmids, miRNA mimics or miRNA antisense oligonucleotides. As shown in Fig. 2a, overexpression of miR-181a markedly downregulated SIRT1 protein levels, whereas overexpression of mutant miR-181a had no significant effect. Moreover, overexpression of miR-181a by its mimics dose-dependently decreased SIRT1 protein levels (Fig. 2b). Consistently, neutralisation of endogenous miR-181a with antisense oligonucleotides dose-dependently elevated SIRT1 protein levels (Fig. 2c). Similarly, in HepG2 cells, transfection with miR-181a mimics or antisense oligonucleotides had similar effects (ESM Fig. 2a,b). More importantly, overexpression of miR-181a markedly downregulated SIRT1 activity and increased the acetylation of two known SIRT1 targets, p53 and histone H3, in HepG2 cells, whereas neutralisation of miR-181a increased SIRT1 activity and decreased the acetylation of p53 and histone H3 (Fig. 2d, ESM Fig. 2a,b). Furthermore, miR-181a had no significant effect on the cellular NAD levels, which contributes to the endogenous deacetylase activity of SIRT1 (ESM Fig. 2d). In addition, neither overexpression nor inhibition of miR-181a changed SIRT1 mRNA levels in both HEK293T cells and HepG2 cells (Fig. 2e,f, ESM Fig. 2c). These data suggest that miR-181a decreases SIRT1 protein levels by inhibiting its translation.
miR-181a downregulates SIRT1 protein abundance at the translational level. a Overexpression of miR-181a but not mutant miR-181a decreased SIRT1 protein levels. After transfection with the indicated plasmids for 72 h, HEK293T cells were harvested for immunoblotting. **p < 0.01 vs pSIF-GFP (pSIF) or pSIF-181a-m expressing mutant miR-181a. b Overexpression of miR-181a dose-dependently downregulated SIRT1 protein levels. After transfection with miR-181a mimics at the indicated concentrations for 72 h, HEK293T cells were harvested for immunoblotting. The doses in nmol/l represent irrelevant miRNA mimics for cel-miR-239b or anti-cel-miR-239b co-transfected as a mock control to equalise the total amount of miRNA mimics or anti-sense oligonucleotides for each transfection; 0 nmol/l represents cells transfected with only cel-miR-239b mimics or anti-cel-miR-239b. **p < 0.01 vs cells treated with only a mock transfection. c Inhibition of miR-181a by antisense oligonucleotides dose-dependently increased SIRT1 protein levels. After transfection with anti-181a at the indicated concentrations for 72 h, HEK293T cells were harvested for immunoblotting. **p < 0.01 vs cells treated with only a mock transfection. d Overexpression of miR-181a decreased SIRT1 activity, whereas inhibition of miR-181a increased SIRT1 activity. After transfection with the indicated oligonucleotides for 72 h, the nuclear protein of HepG2 cells were extracted to measure SIRT1 activity. Black bars, miR-181a; grey bars, anti-181a. **p < 0.01 vs cells treated with only a mock transfection. e Overexpression of miR-181a had no effect on SIRT1 mRNA levels. After transfection with the indicated plasmids for 72 h, HEK293T cells were harvested for real-time PCR. f Overexpression or inhibition of miR-181a had no effect on SIRT1 mRNA levels. After transfection with the indicated oligonucleotides for 72 h, HEK293T cells were harvested for real-time PCR. Black bars, miR-181a; grey bars, anti-181a. Error bars represent SD
miR-181a increases under insulin-resistant conditions
SIRT1, downregulated by miR-181a, has been suggested as a therapeutic target for the prevention of diseases related to insulin resistance [5]. We thus hypothesised that miR-181a levels would be correlated with insulin resistance. To investigate the correlation of miR-181a and insulin resistance, glucosamine-induced insulin resistance in HepG2 cells, a common model for studying insulin resistance, was used [6]. As shown in Fig. 3a,b, glucosamine induced insulin resistance and significant upregulation of miR-181a in HepG2 cells in a time-dependent manner, and the upregulation of miRNA-181a occurred earlier than the decrease in SIRT1 and the appearance of insulin resistance. In addition, we found that glucosamine increased the levels of primary miR-181a, which was significantly attenuated by the transcription inhibitor actinomycin D, suggesting that glucosamine upregulates miR-181a transcription (Fig. 3c). Moreover, under insulin-induced insulin-resistant conditions, the miR-181a level also increased, accompanied by a decrease in SIRT1 protein level (Fig. 3d,e). Consistently, miR-181a was decreased when AICAR, an activator of AMP-activated protein kinase (AMPK), elevated SIRT1 protein levels in HepG2 cells (Fig. 3f,g).
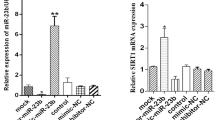
MiR-181a is increased under insulin-resistant conditions and negatively correlated with SIRT1. a Glucosamine upregulated miR-181a level in a time-dependent manner in HepG2 cells. After treatment with glucosamine for the indicated time, HepG2 cells were harvested for real-time PCR. **p < 0.01 vs no treatment. b Glucosamine decreased SIRT1 protein level and induced insulin resistance in HepG2 cells in a time-dependent manner. After treatment with glucosamine for the indicated time, HepG2 cells were stimulated with or without 100 nmol/l insulin for 15 min, and then harvested for immunoblotting. c Glucosamine upregulated the levels of primary miR-181a (pri-181a), and this was significantly attenuated by transcription inhibitor actinomycin D (AMD). HepG2 cells were treated with glucosamine for 18 h to induce insulin resistance (IR) and harvested for real-time PCR. 0.5 mg/ml AMD was added 1 or 3 h before harvesting the cells. Black bars, control; mid-grey bars, glucosamine; dark grey bars, glucosamine + AMD 1 h; light grey bars, glucosamine + AMD 3 h. *p < 0.05, **p < 0.01. d, e Insulin-induced insulin resistance and its correlation with miR-181a and SIRT1 in HepG2 cells. HepG2 cells were treated with 100 nmol/l insulin for 24 h and stimulated with or without insulin for 15 min, before being harvested for real-time PCR or immunoblotting. f, g AICAR significantly elevated SIRT1 protein levels and decreased miR-181a level in HepG2 cells. HepG2 cells were treated with 0.5 mmol/l AICAR for 48 h, and then harvested for real-time PCR or immunoblotting. **p < 0.01. h, i MiR-181a was increased while SIRT1 protein levels were decreased in the liver of db/db mice compared with their littermates. The liver samples from 10-week-old mice with the indicated genotypes were analysed by real-time PCR and immunoblotting. **p < 0.01, n = 14 for each genotype. j, k, l MiR-181a was increased while SIRT1 protein levels and activity were decreased in the livers of DIO mice. Tissues were collected from 16-week-old mice fed chow or a high-fat diet for 10 weeks. **p < 0.01, *p < 0.05, n = 9 for each group. m miR-181a was increased in the serum of diabetic patients. miR-181a levels were detected by real-time PCR, and the relative quantification data were analysed on the log (base 2) scale. **p < 0.01, n = 20 for each group. Error bars represent SD
A similar increase in miR-181a was observed in the livers of db/db mice, a model of type 2 diabetes, where SIRT1 protein levels were decreased (Fig. 3h,i). In addition, miR-181a was also upregulated in the liver of mice fed with a high-fat diet, the content and activity of SIRT1 being decreased, which was further confirmed by the increased acetylation of p53 and histone H3 (Fig. 3j–l). Furthermore, miR-181a was significantly increased in the serum of patients with type 2 diabetes (Fig. 3m).
These results demonstrate that miR-181a increases under conditions of insulin resistance and may be associated with insulin resistance.
Overexpression of miR-181a represses SIRT1 and insulin sensitivity in hepatocytes
To test whether miR-181a regulates insulin sensitivity, we first measured the effect of miR-181a on insulin signalling in hepatic cells. We found that overexpression of miR-181a in HepG2 cells markedly and dose-dependently decreased SIRT1 protein levels and the insulin-stimulated phosphorylation of INSR at Tyr1151, Akt at Ser473 and GSK3β at Ser9 (Fig. 4a,b). Meanwhile, the insulin-stimulated glycogen synthase activity and glycogen synthesis in HepG2 cells were significantly impaired by miR-181a (Fig. 4c,d). To confirm whether miR-181a impairs insulin signalling in other insulin-responsive cells, we overexpressed miR-181a in primary cultured mouse hepatocytes and differentiated C2C12 myotubes. A similar inhibitory effect of miR-181a on SIRT1 and insulin signalling was observed (Fig. 4e,f). In addition, the inhibitory effect of miR-181a on the insulin-stimulated phosphorylation of INSR, Akt, and GSK3β was similar to the effect of Sirt1 siRNA (ESM Fig. 3). Taken together, these data show that miR-181a downregulates SIRT1 protein levels and insulin sensitivity in hepatocytes and C2C12 myotubes.
Overexpression of miR-181a downregulates SIRT1 protein levels and induces insulin resistance. a Overexpression of miR-181a decreased SIRT1 protein levels and the insulin-stimulated phosphorylation of INSR, Akt, and GSK3β in HepG2 cells. After transfection with miR-181a mimics at the indicated concentrations for 72 h, HepG2 cells were stimulated with 100 nmol/l insulin for 15 min and subsequently harvested for immunoblotting. b Quantification of SIRT1 protein levels and insulin-stimulated phosphorylation levels of INSR, Akt and GSK3β corresponding to (a). Black bars, 0 nmol/l; light grey bars, 20 nmol/l; dark grey bars, 40 nmol/l. *p < 0.05, **p < 0.01 vs cells treated with only a mock transfection. c, d Overexpression of miR-181a decreased insulin-stimulated upregulation of glycogen synthase activity (c) and glycogen synthesis (d) in HepG2 cells. Black bars, control; grey bars, insulin. **p < 0.01 vs insulin-stimulated cells treated with only a mock transfection. e, f Overexpression of miR-181a decreased SIRT1 protein levels and the insulin-stimulated phosphorylation of INSR, Akt and GSK3β in primary cultured hepatocytes (e) and C2C12 myotubes (f), respectively. Protein levels were analysed by immunoblotting. Error bars represent SD
Inhibition of endogenous miR-181a increases SIRT1 and insulin sensitivity in hepatocytes under glucosamine-induced insulin-resistant conditions
The induction of insulin resistance by miR-181a leads to the suggestion that inhibition of miR-181a might improve insulin sensitivity. We thus inhibited endogenous miR-181a in HepG2 cells using antisense oligonucleotides to test its effect on insulin sensitivity. Under glucosamine-induced insulin-resistant conditions, inhibition of miR-181a upregulated SIRT1 protein levels and the insulin-stimulated phosphorylation of INSR, Akt and GSK3β (Fig. 5a,b). Furthermore, insulin-stimulated upregulation of glycogen synthase activity and glycogen synthesis were also increased by the inhibition of miR-181a to HepG2 cells under glucosamine-induced insulin-resistant conditions (Fig. 5c,d). These results demonstrate that inhibition of endogenous miR-181a increases SIRT1 protein levels and hepatic insulin sensitivity under glucosamine-induced insulin-resistant conditions.
Inhibition of miR-181a increases SIRT1 protein levels and insulin sensitivity under glucosamine-induced insulin-resistant conditions. a Inhibition of miR-181a by antisense oligonucleotides significantly increased SIRT1 protein levels and the insulin-stimulated phosphorylation of INSR, Akt and GSK3β in HepG2 cells under glucosamine-induced insulin-resistant (IR) condition. After transfection with anti-181a at the indicated concentrations for 72 h, HepG2 cells were stimulated with 100 nmol/l insulin for 15 min and harvested for immunoblotting. b Quantification of SIRT1 protein levels and the phosphorylation levels of INSR, Akt and GSK3β under the glucosamine-induced insulin-resistant conditions in (a). Black bars, 0 nmol/l; light grey bars, 20 nmol/l; dark grey bars, 40 nmol/l. *p < 0.05, **p < 0.01 vs cells in the same group treated only with a mock transfection. c, d Inhibition of miR-181a increased the insulin-stimulated upregulation of glycogen synthase activity (c) and glycogen synthesis (d) in HepG2 cells under glucosamine-induced insulin-resistant conditions. Black bars, control; grey bars, insulin. *p < 0.05, **p < 0.01, vs insulin-stimulated cells treated only with a mock transfection. Error bars represent SD
SIRT1 is involved in the regulation of hepatic insulin sensitivity mediated by miR-181a
Next, we investigated whether SIRT1 is involved in the regulation of hepatic insulin sensitivity by miR-181a. We found that miR-181a successfully inhibited insulin signalling in HepG2 cells infected with control adenovirus (Fig. 6a,b). However, the repressive effect of miR-181a on insulin signalling was greatly attenuated in HepG2 cells infected with adenovirus expressing SIRT1 (Fig. 6a,c). When HepG2 cells were treated with the SIRT1 inhibitor nicotinamide under glucosamine-induced insulin-resistant conditions, inhibition of miR-181a consistently increased SIRT1 protein levels but failed to improve insulin signalling (Fig. 6d,e). Treatment of HepG2 cells with sirtinol, a more specific SIRT1 inhibitor, showed similar results (ESM Fig. 4a). To further confirm the role of SIRT1 in the regulation of insulin sensitivity by miR-181a, primary cultured mouse hepatocytes from Sirt1 knockout mice were used. The repressive effect of miR-181a on the insulin-stimulated phosphorylation of Akt was lost in the primary cultured hepatocytes from Sirt1 null mice (ESM Fig. 4b). Under glucosamine-induced insulin-resistant conditions, the improvement in insulin sensitivity with antisense miR-181a was also markedly attenuated when Sirt1 was absent (Fig. 6f–h). Taken together, these findings indicate that SIRT1 participates in the regulation of hepatic insulin sensitivity mediated by miR-181a.
Regulation of insulin signalling by miR-181a is significantly dependent on SIRT1. a Ectopic expression of SIRT1 by adenovirus (Ad-SIRT1) abrogated the negative effect of miR-181a on the insulin-stimulated phosphorylation of INSR and Akt in HepG2 cells. Ad-Ctrl, control adenovirus. Protein levels were analysed by immunoblotting. b, c Quantification of SIRT1 protein levels and the insulin-stimulated phosphorylation levels of INSR and Akt in HepG2 cells infected with Ad-Ctrl (b) or Ad-SIRT1 (c) in (a). Black bars, 0 nmol/l; light grey bars, 20 nmol/l; dark grey bars, 40 nmol/l. *p < 0.05, **p < 0.01 vs cells in the same group treated with only a mock transfection. d Inhibition of SIRT1 activity by nicotinamide (NAM) abrogated the positive effect of anti-181a on insulin-stimulated phosphorylation of INSR and Akt in HepG2 cells under glucosamine-induced insulin-resistant conditions. Protein levels were analysed by immunoblotting. e Quantification of SIRT1 protein levels and the insulin-stimulated phosphorylation levels of INSR and Akt in HepG2 cells treated with NAM in (d). Black bars, 0 nmol/l; light grey bars, 20 nmol/l; dark grey bars, 40 nmol/l. *p < 0.05, **p < 0.01 vs cells in the same group treated only with a mock transfection. f The improvement in insulin signalling by antisense miR-181a was markedly abrogated in primary hepatocytes of Sirt1 knockout (KO) mice under glucosamine-induced insulin-resistant conditions. g, h Quantification of SIRT1 protein levels and the insulin-stimulated phosphorylation levels of INSR and Akt in primary hepatocytes from wild-type (WT) mice (g) and Sirt1 knockout mice (h) in (f). Black bars, 0 nmol/l; light grey bars, 20 nmol/l; dark grey bars, 40 nmol/l. **p < 0.01 vs cells in the same group treated only with a mock transfection. Error bars represent SD
miR-181a regulates insulin sensitivity and glucose homeostasis in vivo
To investigate whether overexpression of miR-181a also induces insulin resistance in vivo, we use adenovirus to overexpress miR-181a in C57/BL6 mice by tail vein injection. miR-181a levels were markedly upregulated in the mouse liver infected with adenovirus expressing miR-181a precursors (Fig. 7c). Overexpression of miR-181a in mouse liver significantly reduced SIRT1 protein levels and the insulin-induced phosphorylation of INSR, Akt and GSK3β (Fig. 7a,b). In addition, the mRNA levels of lipogenic genes Acc1 and Pparγ (also known as Pparg), and the negative regulators of insulin signalling Ptp1b and Socs3 were significantly increased in the mouse liver with miR-181a overexpression (Fig. 7c). The mRNA and protein levels of the key gluconeogenic genes Pepck and G6pase were also elevated (Fig. 7c,d).
miR-181a regulates SIRT1 and hepatic insulin sensitivity in vivo. a, b Overexpression of miR-181a by adenovirus (Ad-181a) in mouse liver decreased SIRT1 levels and the insulin-stimulated phosphorylation of INSR, Akt and GSK3β. Eight-week-old male C57BL/6 mice were infected with the indicated adenovirus by tail vein injection, and were killed 12 days later to detect by immunoblotting the indicated proteins in liver stimulated with or without insulin. Black bars, control adenovirus (Ad-Ctrl); grey bars, Ad-181a. **p < 0.01 vs Ad-Ctrl, n = 9 for each group in (b). c miR-181a and the mRNA levels of the genes indicated were detected by real-time PCR in mouse liver infected with Ad-181a (grey bars) or Ad-Ctrl (black bars), as described in (a). *p < 0.05, **p < 0.01 vs Ad-Ctrl, n = 6–9 for each group. d The protein levels of G6Pase and phosphoenolpyruvate carboxykinase (PEPCK) were increased in mouse liver infected with Ad-181a when detected by immunoblotting. e, f Intraperitoneal injection of LNA-anti-181a increased SIRT1 protein levels and the insulin-stimulated phosphorylation of INSR, Akt, and GSK3β in the liver of DIO mice. The proteins indicated were measured by immunoblotting (e) and quantified by densitometry (f). Black bars, LNA-control; grey bars, LNA-anti-181a. *p < 0.05 vs LNA-control. g LNA-anti-181a decreased the protein levels of G6pase and PEPCK in the livers of DIO mice when measured by immunoblotting. h MiR-181a and mRNA levels of the genes indicated were detected in the livers of DIO mice injected with LNA-anti-181a (grey bars) or LNA-control (black bars). Real-time PCR was used to analyse these genes. *p < 0.05, **p < 0.01 vs LNA-control, n = 9–11 for each group. i, j LNA-anti-181a improved glucose tolerance in DIO mice as determined by a glucose tolerance test (GTT) (i). White diamonds, chow PBS; black circles, high-fat diet+PBS; white triangles, high-fat diet+LNA-control; black squares, high-fat diet+LNA-anti-181a. The area under the curve (AUC) of GTT was also reduced by LNA-anti-181a (j) White bars, chow+PBS; light grey bars, high-fat diet+PBS; dark grey bars, high-fat diet+LNA-control; black bars, high-fat diet+LNA-anti-181a. *p < 0.05, **p < 0.01 vs LNA-control, n = 9–13 for each group. k LNA-anti-181a decreased the 4-h fasting blood glucose levels in DIO mice. White bar, chow+PBS; light grey bar, high-fat diet+PBS; dark grey bar, high-fat diet+LNA-control; black bar, high-fat diet+NA-anti-181a. *p < 0.05, **p < 0.01 vs LNA-control, n = 9–13 for each group. l, m Individual correlation between hepatic miR-181a levels and 4-h fasting blood glucose levels (l; R 2 = 0.58, p = 0.006) or the AUC of GTT (m; R 2 = 0.63, p = 0.003) in the DIO mice injected with LNA-anti-181a (n = 11). Statistical significance was assessed by Pearson correlation coefficient test. Error bars represent SD
We then detected whether inhibition of endogenous miR-181a could improve insulin sensitivity in vivo. After intraperitoneal injection of LNA antisense oligonucleotides for miR-181a (LNA-anti-181a), miR-181a levels in the liver of DIO mice were markedly reduced (Fig. 7h). Moreover, inhibition of miR-181a by LNA-anti-181a significantly increased SIRT1 protein levels and the insulin-induced phosphorylation of INSR, Akt and GsSK3β in both the liver and muscle of DIO mice (Fig. 7e,f, ESM Fig. 5a). Meanwhile, hepatic protein and mRNA levels of Pepck and G6pase were both significantly decreased in the LNA-anti-181a-treated DIO mice (Fig. 7g,h). In addition, the hepatic mRNA levels of Acc1, Pparγ and Ptp1b were significantly decreased in the LNA-anti-181a-treated DIO mice (Fig. 7h). In the muscle of LNA-anti-181a-treated DIO mice, mRNA levels of Foxo1, Glut4 and Pgc1α were increased (ESM Fig. 5b). The alteration in these genes is consistent with the improved insulin signalling in liver and muscle (Fig. 7e,f, ESM Fig. 5a). In agreement with the improved insulin sensitivity in liver and muscle and the downregulation of key hepatic gluconeogenic enzyme genes induced by LNA-anti-181a, we found that LNA-anti-181a significantly downregulated 4-h fasting blood glucose levels and improved glucose tolerance in the DIO mice (Fig. 7i–k). Moreover, there was a positive correlation between the hepatic miR-181a levels and the fasting blood glucose, as well as the areas under the curve of glucose tolerance tests, in the DIO mice injected with LNA-anti-181a (Fig. 7l,m). Taken together, these data show that inhibition of miR-181a ameliorates DIO-induced hepatic insulin resistance and improves glucose homeostasis in DIO mice.
Discussion
In this study, we identified SIRT1 as a direct target of miR-181a and demonstrated that miR-181a negatively regulates SIRT1 and hepatic insulin sensitivity. These results suggest that miR-181a might represent a potential therapeutic target for the treatment of insulin resistance and type 2 diabetes.
Recently, a study reported that miR-9, miR-135, miR-199b, miR-204, miR-181a and miR-181b reduce endogenous SIRT1 in mouse embryonic stem cells [29]. In our study, we further demonstrate that miR-181a downregulates SIRT1 protein levels in hepatocytes and muscle cells. Moreover, our study also showed that miR-181a decreases SIRT1 protein levels in mouse liver. Previous studies have shown that SIRT1 can be regulated by multiple miRNAs, such as miR-9, miR-22, miR-34a, miR-132, miR-199a and miR-217 in some different biological processes [27, 30–32]. In our experiment, we similarly observed the inhibitory effect of these miRNAs. Moreover, we also discovered that miR-543 and miR-30a could significantly decrease the activity of Sirt1 3′UTR in HEK293T cells. miR-30a was found to reduce autophagic activity, which is positively regulated by SIRT1 [33, 34], suggesting that miR-30a may regulate autophagy by downregulating SIRT1. The distinct biological functions of these miRNAs targeting SIRT1 need to be further explored. In our study, although no obvious repressive effects of miR-22, miR-34a, miR-132 or miR-217 on Sirt1 3′UTR were observed in HEK293T cells, we did find their effects on Sirt1 3′UTR in HepG2 cells, suggesting that miRNAs may play different roles in different cells. Taken together, these findings suggest that SIRT1 is regulated by various miRNAs to function diversely in specific cells and tissues.
Many studies have investigated the function of miR-181a in tissues where it is highly expressed, such as thymus, brain and spleen [35, 36]. It has also been reported that miR-181a is a critical player in epithelial cell adhesion molecule (EpCAM)-positive hepatic cancer stem cells by targeting transcriptional regulators of differentiation [37]. However, the biological function of miR-181a in liver is still poorly understood. Here we demonstrate for the first time that miR-181a is an important regulator in hepatic insulin sensitivity. Overexpression of miR-181a induces hepatic insulin resistance, whereas inhibition of endogenous miR-181a improves hepatic insulin sensitivity both in vitro and in vivo. Remarkably, inhibition of miR-181a by LNA-anti-181a improves glucose homeostasis in DIO mice. The efficacy and safety of LNA antisense oligonucleotides in inhibiting miRNA targets have been shown in mouse and non-human primate models [38, 39]. Several different LNA drugs are currently in clinical trials against cancer and infectious diseases [40]. LNA-anti-181a might thus have potential therapeutic value for treating insulin resistance and type 2 diabetes. In addition, we found that miR-181a significantly increased in the serum of patients with type 2 diabetes, implying that serum miR-181a is a potential diagnostic marker, similar to other miRNAs described previously [41].
Consistent with our results, knockdown of SIRT1 impairs insulin signalling in HepG2 hepatoma cells [6]. Liver-specific Sirt1 knockout mice display signs of hepatic insulin resistance, and exhibit significantly increased fasting glucose levels when fed with a high-fat diet [42]. Hepatic SIRT1 deficiency in mice impairs mammalian target of rapamycin complex 2 (mTORC2)/Akt signalling and results in hyperglycaemia and insulin resistance [26]. Furthermore, adenovirus-mediated overexpression of SIRT1 in the liver of ob/ob mice and of LDL receptor-deficient mice fed a high-fat high-sugar diet attenuates hepatic steatosis and ameliorates systemic insulin resistance [9]. Similarly, our data show that inhibition of miR-181a by LNA-anti-181a is sufficient to improve hepatic insulin sensitivity accompanied by upregulation of SIRT1 protein levels in DIO mice.
Since a single vertebrate miRNA may target up to approximately 100–200 mRNAs [43], it is likely that some other miR-181a targets participate in the regulation of hepatic insulin sensitivity. SHP2, a tyrosine phosphatase, regulates insulin signalling positively and has been shown to be repressed by miR-181a in T cells [44, 45]. However, we found that protein levels of SHP2 were not changed in HepG2 cells or the liver of mice when miR-181a was overexpressed (data not shown). RNA-binding protein LIN-28 homologue A, another target of miR-181a, has also been reported as a positive regulator of insulin signalling [46, 47]. Foxo1 and Pgc1α were also predicted as miR-181a targets by PicTar or TargetScan. Whether these genes are involved in miR-181a-regulated hepatic insulin sensitivity needs to be investigated. In this study, we found that inhibition of SIRT1 activity or SIRT1 deficiency abrogated the positive effect of antisense miR-181a on insulin signalling, and overexpression of SIRT1 could sufficiently improve the insulin resistance induced by miR-181a. These data suggest that miR-181a regulates insulin sensitivity at least mainly through SIRT1. However, whether the effect of miR-181a on insulin sensitivity is SIRT1-dependent in vivo also needs to be studied.
SIRT1 has been reported as a positive regulator of insulin signalling by targeting protein tyrosine phosphatase 1B (PTP1B), AMPK, forkhead box O1 (FOXO1), peroxisome proliferator-activated receptor γ coactivator 1α (PGC1Α), and so on [5]. Our in vivo experiments show that some glucose and lipid metabolism-related genes in liver or muscle, including Ptp1b, G6pase, Pepck, Acc1, Foxo1, Pgc1α and Glut4, are regulated by miR-181a. The alteration in these genes might be part of the underlying mechanisms by which miR-181a regulates insulin signalling through SIRT1. Besides miR-181a, miR-22 and miR-34a were also found to be elevated in the liver of diabetic db/db mice [48]. Whether miR-22 and miR-34a can regulate insulin sensitivity through SIRT1 needs to be investigated.
In summary, our results indicate that miR-181a regulates hepatic insulin sensitivity and that SIRT1 is involved in this process. Inhibition of miR-181a is capable of improving hepatic insulin sensitivity and glucose homeostasis, which might provide a potential new therapeutic strategy for treating insulin resistance and type 2 diabetes.
Abbreviations
- 3′UTR:
-
3′ Untranslated region
- DIO:
-
Diet-induced obesity
- GSK3β:
-
Glycogen synthase kinase 3β
- INSR:
-
Insulin receptor
- LNA:
-
Locked nucleic acid
- miRNA:
-
MicroRNA
- PI3K:
-
Phosphoinositide 3-kinase
- siRNA:
-
Small interfering RNA
- SIRT1:
-
Sirtuin-1
- SHP2:
-
SH2 domain-containing protein tyrosine phosphatase 2
References
Yach D, Stuckler D, Brownell KD (2006) Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med 12:62–66
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444:840–846
Guarente L (2006) Sirtuins as potential targets for metabolic syndrome. Nature 444:868–874
Haigis MC, Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 5:253–295
Liang F, Kume S, Koya D (2009) SIRT1 and insulin resistance. Nat Rev Endocrinol 5:367–373
Sun C, Zhang F, Ge X et al (2007) SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6:307–319
Yoshizaki T, Milne JC, Imamura T et al (2009) SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 29:1363–1374
Frojdo S, Durand C, Molin L et al (2011) Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol 335:166–176
Li Y, Xu S, Giles A et al (2011) Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J 25:1664–1679
Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH (2008) Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A 105:9793–9798
Banks AS, Kon N, Knight C et al (2008) SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 8:333–341
Baur JA, Pearson KJ, Price NL et al (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342
Milne JC, Lambert PD, Schenk S et al (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450:712–716
Feige JN, Lagouge M, Canto C et al (2008) Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8:347–358
Lagouge M, Argmann C, Gerhart-Hines Z et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127:1109–1122
Kloosterman WP, Plasterk RH (2006) The diverse functions of microRNAs in animal development and disease. Dev Cell 11:441–450
Pandey AK, Agarwal P, Kaur K, Datta M (2009) MicroRNAs in diabetes: tiny players in big disease. Cell Physiol Biochem 23:221–232
Teleman AA, Maitra S, Cohen SM (2006) Drosophila lacking microRNA miR-278 are defective in energy homeostasis. Genes Dev 20:417–422
Herrera BM, Lockstone HE, Taylor JM et al (2010) Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia 53:1099–1109
Zhao E, Keller MP, Rabaglia ME et al (2009) Obesity and genetics regulate microRNAs in islets, liver, and adipose of diabetic mice. Mamm Genome 20:476–485
Huang B, Qin W, Zhao B et al (2009) MicroRNA expression profiling in diabetic GK rat model. Acta Biochim Biophys Sin (Shanghai) 41:472–477
Ling HY, Ou HS, Feng SD et al (2009) Changes in microRNA profile and effects of miR-320 in insulin-resistant 3 T3-L1 adipocytes. Clin Exp Pharmacol Physiol 36:e32–e39
He A, Zhu L, Gupta N, Chang Y, Fang F (2007) Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3 T3-L1 adipocytes. Mol Endocrinol 21:2785–2794
Pandey AK, Verma G, Vig S, Srivastava S, Srivastava AK, Datta M (2011) miR-29a levels are elevated in the db/db mice liver and its overexpression leads to attenuation of insulin action on PEPCK gene expression in HepG2 cells. Mol Cell Endocrinol 332:125–133
Li ZY, Na HM, Peng G, Pu J, Liu P (2011) Alteration of microRNA expression correlates to fatty acid-mediated insulin resistance in mouse myoblasts. Mol Biosyst 7:871–877
Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX (2011) Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest 121:4477–4490
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 105:13421–13426
Stumvoll M, Goldstein BJ, van Haeften TW (2005) Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365:1333–1346
Saunders LR, Sharma AD, Tawney J et al (2010) miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2:415–431
Menghini R, Casagrande V, Cardellini M et al (2009) MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 120:1524–1532
Rane S, He M, Sayed D et al (2009) Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res 104:879–886
Strum JC, Johnson JH, Ward J et al (2009) MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol 23:1876–1884
Zhu H, Wu H, Liu XP et al (2009) Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy 5:816–823
Lee IH, Cao L, Mostoslavsky R et al (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105:3374–3379
Sonkoly E, Stahle M, Pivarcsi A (2008) MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol 18:131–140
Shi L, Cheng Z, Zhang J et al (2008) hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res 1236:185–193
Ji J, Yamashita T, Budhu A et al (2009) Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology 50:472–480
Elmen J, Lindow M, Schutz S et al (2008) LNA-mediated microRNA silencing in non-human primates. Nature 452:896–899
Elmen J, Lindow M, Silahtaroglu A et al (2008) Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res 36:1153–1162
Lanford RE, Hildebrandt-Eriksen ES, Petri A et al (2010) Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327:198–201
Mitchell PS, Parkin RK, Kroh EM et al (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 105:10513–10518
Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X (2009) Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 9:327–338
Krek A, Grun D, Poy MN et al (2005) Combinatorial microRNA target predictions. Nat Genet 37:495–500
Princen F, Bard E, Sheikh F et al (2009) Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death. Mol Cell Biol 29:378–388
Li QJ, Chau J, Ebert PJ et al (2007) miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 129:147–161
Li X, Zhang J, Gao L et al (2012) MiR-181 mediates cell differentiation by interrupting the Lin28 and let-7 feedback circuit. Cell Death Differ 19:378–386
Zhu H, Shyh-Chang N, Segre AV et al (2011) The Lin28/let-7 axis regulates glucose metabolism. Cell 147:81–94
Kaur K, Pandey AK, Srivastava S, Srivastava AK, Datta M (2011) Comprehensive miRNome and in silico analyses identify the Wnt signaling pathway to be altered in the diabetic liver. Mol Biosyst 7:3234–3244
Acknowledgement
The authors thank M. W. McBurney (Center for Cancer Therapeutics, Ottawa Hospital Research Institute) for providing the Sirt1 +/− mice.
Funding
This work was supported by grants from National Natural Science Foundation of China (30825009, 30970619, 31030022 and 81021002), National Basic Research Program of China (973 Program, 2009CB918403 and 2007CB914501), Director Foundation of Institute for Nutritional Sciences (20090101), SA-SIBS Scholarship Program, China Postdoctoral Science Foundation (20100480641), National Science and Technology Support Program (2009BAI80B04), The Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-R-09), the Postdoctoral Research Program of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (2011KIP511), Program of Shanghai Subject Chief Scientist (11XD1405800) and Shanghai Postdoctoral Scientific Program (11R21417400) and Shanghai Xuhui Central Hospital.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
This study was designed by BZ, CL and QWZ. The experiments were performed by BZ, CL, WQ, YZ, FZ, JXW, YNH, DMW, YL and TTY. QJ and MFL contributed to materials, methods and discussion. BZ, CL and QWZ analysed the data. QWZ supervised the project. CL, BZ and QWZ wrote the paper. All authors participated in data interpretation and revising the paper, and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Zhou and C. Li contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 191 kb)
ESM Fig. 2
(PDF 108 kb)
ESM Fig. 3
(PDF 167 kb)
ESM Fig. 4
(PDF 54 kb)
ESM Fig. 5
(PDF 176 kb)
ESM Table 1
(PDF 127 kb)
ESM Methods
(PDF 129 kb)
Rights and permissions
About this article
Cite this article
Zhou, B., Li, C., Qi, W. et al. Downregulation of miR-181a upregulates sirtuin-1 (SIRT1) and improves hepatic insulin sensitivity. Diabetologia 55, 2032–2043 (2012). https://doi.org/10.1007/s00125-012-2539-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2539-8