Abstract
Aims/hypothesis
In type 2 diabetic patients at low risk for cardiovascular disease (CVD), the relationship between the clinical course of nephropathy by stage of chronic kidney disease (CKD) and onset of CVD remains unclear. Clarification of this relationship is important for clinical decision-making for both low- and high-risk diabetic patients.
Methods
This 4 year prospective study enrolled 2,954 type 2 diabetic patients with no prevalent CVD, and serum creatinine <176.8 μmol/l. The risk for CVD onset (non-fatal and fatal CVD and stroke, and peripheral arterial disease) was assessed according to CKD stage categorised by urinary albumin-to-creatinine ratio (ACR; mg/mmol) and estimated GFR (eGFR; ml min−1 1.73 m−2). Association of progression from ‘no CKD’ stage (ACR <3.5 mg/mmol and eGFR ≥90 ml min−1 1.73 m−2) with risk for CVD onset was also evaluated.
Results
During follow-up (median 3.8 years), 89 CVD events occurred. Compared with patients with ‘no CKD’ as reference, those with ACR ≥ 35.0 mg/mmol with co-existing eGFR 60–89 ml min−1 1.73 m−2 or <60 ml min−1 1.73 m−2 showed increased risk for CVD onset, whereas those with eGFR ≥90 ml min−1 1.73 m−2 did not. Those with ACR <3.5 mg/mmol and eGFR <60 ml min−1 1.73 m−2 did not show any increased risk. Among patients with ‘no CKD’ stage at baseline, those who progressed to ACR ≥3.5 mg/mmol during follow-up showed an increased risk compared with those who did not, whereas those who progressed to eGFR <90 ml min−1 1.73 m−2 did not have increased risk.
Conclusions/interpretation
The risk for CVD was associated with progression of albuminuria stage rather than eGFR stage in type 2 diabetic patients at relatively low risk for CVD.
Similar content being viewed by others
Introduction
Kidney Disease Improving Global Outcomes (KDIGO) has proposed a new classification for chronic kidney disease (CKD) categorised by the stages of estimated GFR (eGFR) and albuminuria [1]. Diabetic nephropathy is likely to be applicable to this category, and through this category renal–cardiovascular risk should be estimated. In patients with type 2 diabetes mellitus at relatively high risk for cardiovascular disease (CVD) (i.e. white populations including those with prevalent CVD), there are several prospective studies [2–4], some of which have shown an independent association of decreased eGFR and increased albuminuria with renal–cardiovascular outcomes [3, 4]. However, the contribution of eGFR and albuminuria to CVD risk may differ between patients at high risk and low risk for CVD. Studies on low-risk populations are therefore required.
The aim of the present study was to investigate the association between eGFR and albuminuria categories and CVD risk in Japanese patients with type 2 diabetes but without a history of CVD and with serum creatinine (Cr) <176.8 μmol/l (2.0 mg/dl)—a group considered to be at relatively low risk for CVD—by using the data from a large nationwide prospective cohort study, the Japan Diabetes Clinical Data Management (JDDM) study.
Methods
Study population
The JDDM study is a large ongoing nationwide prospective cohort study performed in primary care settings. A detailed description of the cohort has been published previously [5–7]. Briefly, 3,611 Japanese patients with diabetes mellitus who were aged between 20 and 70 years and were treated for diabetes more than 1 year in one of 17 centres were assessed in 2004. The backgrounds of these potential participants were not different from those of the individuals included in the JDDM study overall (n = 16,394) in terms of clinical characteristics [8].
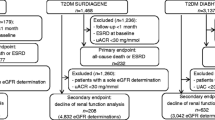
Patients with type 1 diabetes (n = 288), serum Cr ≥176.8 μmol/l (n = 39), or prevalent CVD (n = 296) were excluded, as were those who did not agree to participate (n = 4) and those for whom baseline data of urinary albumin-to-creatinine ratio (ACR) or serum Cr were unavailable (n = 31). A total of 2,954 individuals with type 2 diabetes were subsequently enrolled in this cohort study. Prevalent CVD at baseline included a history of ischaemic stroke, CHD and/or peripheral arterial disease (PAD) diagnosed by each physician based on medical records, as previously described in detail [6]. Approval for the cohort study was obtained from the ethical committee of the JDDM study and each centre, and all participants provided written informed consent.
Measurements and definition
Type 2 diabetes was defined according to the Japan Diabetes Society (JDS) criteria [9]. BP was measured in the sitting position after the participant had rested for more than 5 min, and the average of three measurements on different days was recorded. Hypertension was defined as BP ≥140/90 mmHg or current use of antihypertensive drugs. Non-fasting blood samples were obtained for measurements of HbA1c and serum concentrations of Cr and lipids, which were performed at local laboratories. HbA1c was measured by high-performance liquid chromatography, which was standardised by the JDS (normal range, 4.3–5.8%) and was certified by the US National Glycohemoglobin Standardization Program (NGSP; NGSP = JDS + 0.40) [9]. Dyslipidaemia was defined as: serum concentration of total cholesterol ≥5.7 mmol/l, triacylglycerol ≥1.7 mmol/l or HDL-cholesterol <1.0 mmol/l (<1.2 mmol/l if female); or patient already receiving lipid-lowering agents. LDL-cholesterol levels were calculated by Friedewald’s formula. Serum and urinary concentrations of Cr were measured by an enzymatic method.
Three random urine samples were obtained at baseline, and urinary albumin was measured by a turbidimetric immunoassay at each laboratory. According to the new classification of CKD, the albuminuria category was classified by two of three samples at baseline being in the stages ACR <3.5 mg/mmol (<30 mg/g, normoalbuminuria), ACR ≥3.5 and <35.0 mg/mmol (≥30 and <300 mg/g, microalbuminuria) and ACR ≥35.0 mg/mmol (≥300 mg/g, overt proteinuria). eGFR was estimated using an equation proposed by the Japanese Society of Nephrology [10]. The new Japanese equation is reasonably accurate in estimating GFR for the Japanese population and is more accurate than both the modified Modification of Diet in Renal Disease equation refitted for the Japanese by overcoming the underestimation of GFR at high values up to 110 ml min−1 1.73 m−2 [10], and the CKD Epidemiology Collaboration (CKD-EPI) equation modified for the Japanese population [11]. The mean of two consecutive measures of eGFR in the first 4 months was used as the baseline eGFR value for each patient. CKD stages were classified by three albuminuria and eGFR categories: ACR <3.5, 3.5-34.9 and ≥35.0 mg/mmol; and eGFR ≥90, 60–89, and <60 ml min−1 1.73 m−2. In this study, the term ‘no CKD’ was defined as normoalbuminuria (ACR < 3.5 mg/mmol) and eGFR ≥90 ml min−1 1.73 m−2. Diagnostic criteria for diabetic retinopathy and neuropathy are published elsewhere [6].
Follow-up and outcomes
Patients attended the clinic monthly or bimonthly and were followed-up until the end of 2008 or the first occurrence of one of the following endpoints: non-fatal CHD, non-fatal ischaemic stroke, PAD or death. Treatment goals recommended by the JDS were HbA1c <6.9% (52 mmol/mol) (NGSP), BP <130/80 mmHg, and serum concentrations of total cholesterol <5.2 mmol/l (200 mg/dl), triacylglycerol <1.7 mmol/l (150 mg/dl), HDL-cholesterol >1.0 mmol/l (40 mg/dl) and BMI 20–24 kg/m2.
The main outcome of this study was the new onset of CVD: non-fatal CHD, non-fatal ischaemic stroke, PAD or cardiovascular death. These corresponded to the International Classification of Diseases, 10th revision, codes (www.who.int/classifications/icd/en/) I20-21, I63 and I70. The composite outcome included CVD plus all-cause death. Definitions for CHD, ischaemic stroke and PAD are given elsewhere [7]. We classified all fatal events as cardiovascular unless there was a clear non-cardiovascular cause. An independent panel, working with the endpoint adjudication committee, assessed all potential endpoints and classified them in accordance with predefined criteria.
In addition, progression and regression of albuminuria and eGFR stage during the follow-up were explored. ACR and eGFR were measured three times a year and the last three and two measures prior to the endpoint, respectively, were used. Staging in albuminuria and eGFR at the end of follow-up was performed using the same definition as at baseline.
Statistical analysis
Data are expressed as mean±SD or median (interquartile range). For comparison between two groups, the unpaired Student’s t test was used for normally distributed variables, and the Mann–Whitney U test for variables with skewed distribution. Statistical significance of the differences among the three groups was determined by χ 2 tests for categorical variables and ANOVA for continuous variables. Incidence rates of CVD events and composite endpoints for different categories were calculated. Cox proportional hazards analysis was used to compute hazard ratios and 95% CIs to assess the effect of albuminuria and eGFR on the outcomes using the ‘no CKD’ group as a reference.
In multivariate analysis, adjustments for cardiovascular risk factors included age, sex, hypertension, dyslipidaemia, smoking and BMI. The p value for trend by albuminuria was explored using trend tests in the Cox model after adjustment for cardiovascular risk factors and eGFR strata, and vice versa for the trend by eGFR.
To explore the effect of progression of albuminuria and eGFR on the risk for CVD in patients with ‘no CKD’ stage, worsening of albuminuria stage (ACR ≥3.5 mg/mmol) and eGFR stage (eGFR <90 ml min−1 1.73 m−2) were used in categorical analysis (model 1). In the categorical analysis, patients with an ACR or eGFR value close to the boundary for each stage might frequently progress to next stage because of random measurement error. To minimise this inherent problem, we also used the following alternative definition in a continuous analysis (model 2): progression of albuminuria was defined as ACR ≥3.5 mg/mmol and 50% increase in the geometric mean of ACR from baseline and progression of eGFR was defined as the annual change of eGFR ≤ −4% per year [12].
The hazard ratio and 95% confidence interval are given. A p value under 5% (two-tailed) was considered to be significant. All analyses were performed with the statistical software package SPSS (SPSS Japan, Tokyo, Japan).
Results
Baseline characteristics
Among the participants, 180 individuals (6.1%) were lost to follow-up while free of any event, and the remaining 2,774 (93.9%) were followed until the onset of a first event or the end of the observation period. The former patients were distributed equally with respect to CKD stage. Table 1 shows the baseline characteristics of 2,954 participants by albuminuria stage. Variables that increased according to the progression in albuminuria stage were BMI, duration of diabetes, insulin treatment, HbA1c, hypertension, systolic BP, dyslipidaemia, total cholesterol, triacylglycerol, smoking, microvascular complications and low eGFR stage.
Incidence rate of events stratified by CKD stage
During a median follow-up of 3.8 years (interquartile range 3.1 to 4.3 years), 89 CVD events occurred (46 CHD, 36 ischaemic stroke, seven with PAD), including five CVD deaths, and 30 non-cardiovascular deaths occurred. The incidence rates (per 1,000 person-years) of CVD, CHD and ischaemic stroke in all participants was 8.3, 4.3 and 3.3, respectively. The incidence rates of CVD and composite endpoints increased with worsening of albuminuria stage and eGFR stage (Table 2). In particular, high incidence rates were observed in those with albuminuria stages >35.0 mg/mmol plus co-existing eGFR of 60–89 or <60 ml min−1 1.73 m−2.
Risk for cardiovascular events stratified by CKD stage
The risk for CVD and composite endpoints stratified by CKD stage was evaluated by a Cox hazards model after adjustment for cardiovascular risk factors (Table 3). The effects of albuminuria and eGFR stages were independent of each other (p > 0.50 for interaction). The hazard ratios were significantly high for albuminuria stages of >35.0 mg/mmol plus a co-existing eGFR of 60–89 or <60 ml min−1 1.73 m−2 (p < 0.05). Across all eGFR groups, albuminuria stage significantly increased the adjusted hazard ratio (p = 0.002). However, across all albuminuria groups, eGFR stage did not increase it.
Transition of the stages in albuminuria and eGFR
The proportions of patients transiting to the next stage of albuminuria or eGFR during the follow-up are shown by the CKD categories in Fig. 1a,b. The analyses were performed in 2,501 patients whose data for ACR and eGFR were available at the end of follow-up; the stage was determined by multiple measurements of ACR and eGFR, as described in “Methods” section. The proportions of progression and regression in albuminuria stage were similar at any eGFR stage, and the regression was constantly higher than the progression. Progression from eGFR ≥90 ml min−1 1.73 m−2 to a lower level was high, at around 40%. The proportion of progression of eGFR increased according to worsening albuminuria stage, with annual rates of eGFR decline of −0.7 ± 4.2, −1.9 ± 4.7, and −7.1 ± 9.9% per year in groups with ACR <3.5, 3.5-34.9 and ≥35.0 mg/mmol, respectively (p < 0.0001 by ANOVA).
Progression of albuminuria and eGFR in association with CVD onset among ‘no CKD’ patients
Among patients with ‘no CKD’, patients who progressed to albuminuria stage ≥3.5 mg/mmol showed higher baseline values in HbA1c (7.7 ± 1.1 vs 7.5 ± 1.0%, 61 ± 9 vs 58 ± 8 mmol/mol, p < 0.001) and ACR (2.1 [1.5 to 2.7] vs 1.1 [0.7 to 1.7] mg/mmol, p < 0.001), lower values in HDL-cholesterol (1.36 ± 0.38 vs 1.44 ± 0.41 mmol/l, p < 0.05), and higher proportions of hypertension (48% vs 36%) and dyslipidaemia (69% vs 56%) compared with those who remained in albuminuria stage <3.5 mg/mmol. Consequently, the incidence and adjusted hazard ratios for CVD onset were higher in patients who progressed to ACR ≥3.5 mg/mmol than in those who did not (Table 4, model 1 as categorical analysis). Model 2 continuous analysis gave similar results. In contrast, no differences in risk for CVD were found between those with and without progression of eGFR.
Discussion
In Japanese patients with type 2 diabetes but without any history of CVD, not only those with ACR ≥35.0 mg/mmol and eGFR <60 but also those with ACR ≥35.0 mg/mmol and eGFR 60–89 ml min−1 1.73 m−2 had a high risk for CVD, whereas those with ACR <3.5 mg/mmol did not show any increased risk, even with eGFR <60 ml min−1 1.73 m−2. Furthermore, among patients with ‘no CKD’ stage, an increased risk for CVD was found in those who developed ACR ≥3.5 mg/mmol but not in those who developed eGFR <90 ml min−1 1.73 m−2. To our knowledge, this is the first report to show the association of the clinical course of albuminuria and eGFR stage with the onset of CVD in patients with type 2 diabetes at relatively low risk for CVD.
Association of albuminuria and eGFR with CVD risk
Our cohort indicated that normoalbuminuric CKD was not a high-risk factor for CVD, which is consistent with other studies of diabetic and non-diabetic populations [2, 13, 14]. On the other hand, the Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation (ADVANCE) and Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) studies reported an increased risk of reduced eGFR for CVD independent of albuminuria. The backgrounds of the study populations were different, with recruitment year, age, diabetes duration, proportion of previous history of CVD, and incidence of CVD per 1,000 person-years: 1998, 66 years, 7 years, 32% and 20.5 in the ADVANCE study [3]; 1998, 62 years, 5 years, 22% and 26.4 in the FIELD study [4]; and 2004, 58 years, 11 years, 0% and 8.3 in the JDDM study. Participants in the first two studies were apparently at high risk for CVD. The low incidence in the JDDM study may be attributed to the multifactorial approach for cardiovascular risk used in this recently recruited cohort [15, 16], coupled with the ethnicity and exclusion of those with CVD. It is likely that the effects of albuminuria and eGFR on risk for CVD are somewhat different between those at low risk and high risk for CVD.
Microalbuminuria and CVD risk
Patients who developed microalbuminuria showed a significantly higher risk for CVD than those who did not, with a similar finding in the FIELD study [4]. The reason why patients with microalbuminuria did not show a significant risk for CVD may be that the risk for CVD in those with normoalbuminuria was overestimated by including patients who subsequently developed microalbuminuria. Even within the normoalbuminuric range, patients who developed CVD had higher levels of baseline ACR than those who did not, and this finding is supported by other studies [6, 17–19]. Taken together, preventing the development of microalbuminuria appears to be an important objective for preventing CVD in patients with type 2 diabetes at low risk as well as those at high risk.
Considering the transition in ACR and eGFR stages
The regression in albuminuria stage was consistently higher than the progression at any level of eGFR stage. Only a few studies have reported on the transition of albuminuria stage [20–22], and no studies have done so in association with eGFR level. This cohort provided clinically interesting evidence that reducing albuminuria is possible even in patients with eGFR <60 ml min−1 1.73 m−2. Moreover, the progression rate in eGFR increased according to worsening in albuminuria stages, indicating the importance of reduction of albuminuria.
Study limitation
We acknowledge that the low incidence of CVD giving a wide CI might lead to a potential lack of statistical power. However, strengths of the study include the low dropout rate and multiple measures of eGFR and ACR, both at baseline and follow-up. These are likely to increase the accuracy of risk estimation.
In conclusion, the risk for CVD was associated with progression in albuminuria stage rather than eGFR stage in Japanese patients with type 2 diabetes and no prevalent CVD, suggesting that the effect of albuminuria and eGFR on CVD risk may be somewhat different between those at low risk and high risk for CVD.
Abbreviations
- ACR:
-
Urinary albumin-to-creatinine ratio
- ADVANCE:
-
Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation
- CKD:
-
Chronic kidney disease
- Cr:
-
Creatinine
- CVD:
-
Cardiovascular disease
- eGFR:
-
Estimated GFR
- FIELD:
-
Fenofibrate Intervention and Event Lowering in Diabetes
- JDDM:
-
Japan Diabetes Clinical Data Management
- JDS:
-
Japan Diabetes Society
- NGSP:
-
National Glycohemoglobin Standardization Program
- PAD:
-
Peripheral arterial disease
References
Levey AS, de Jong PE, Coresh J et al (2011) The definition, classification and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 80:17–28
Bruno G, Merletti F, Bargero G et al (2007) Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia 50:941–948
Ninomiya T, Perkovic V, de Galan BE, ADVANCE Collaborative Group et al (2009) Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20:1813–1821
Drury PL, Ting R, Zannino D et al (2011) Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 54:32–43
Yokoyama H, Kawai K, Oishi M, Sone H, on behalf of Japan Diabetes Data Management Study Group (2008) Familial predisposition to cardiovascular risk and disease contributes to cardiovascular risk and disease interacting with other cardiovascular risk factors in diabetes-implication for common soil (JDDM14). Atherosclerosis 201:332–338
Yokoyama H, Oishi M, Kawai K, Sone H, on behalf of the Japan Diabetes Clinical Data Management Study Group (2008) Reduced GFR and microalbuminuria are independently associated with prevalent cardiovascular disease in type 2 diabetes: JDDM study 16. Diabetic Med 25:1426–1432
Yokoyama H, Matsushima M, Kawai K, on behalf of Japan Diabetes Clinical Data Management Study Group et al (2011) Low incidence of cardiovascular events in Japanese patients with type 2 diabetes in primary care settings: a prospective cohort study. Diabetic Med 28:1221–1228
Kobayashi M, Yamazaki K, Hirao K et al (2006) The status of diabetes control and antidiabetic drug therapy in Japan—a cross-sectional survey of 17,000 patients with diabetes mellitus (JDDM 1). Diabetes Res Clin Pract 73:198–204
Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus (2010) The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. J Diabetes Invest 1:212–228
Matsuo S, Imai E, Horio M et al (2009) Collaborators Developing the Japanese Equation for Estimated GFR: revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S (2010) Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis 56:32–38
Yokoyama H, Kanno S, Takahashi S et al (2011) Risks for glomerular filtration rate decline in association with progression of albuminuria in type 2 diabetes. Nephrol Dial Transplant 26:2924–2930
Rigalleau V, Lasseur C, Raffaitin C et al (2007) Normoalbuminuric renal insufficient diabetic patients: a lower-risk group. Diabetes Care 30:2034–2039
Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT, PREVEND Study Group (2008) Cardiovascular and renal outcome in subjects with K/DOQI stage 1–3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant 23:3851–3858
Lutgers HL, Gerrits EG, Sluiter WJ et al (2009) Life expectancy in a large cohort of type 2 diabetes patients treated in primary care (ZODIAC-10). PLoS One 28:e6817
Oluwatowoju I, Abu E, Wild SH et al (2010) Improvements in glycaemic control and cholesterol concentrations associated with the Quality and Outcomes Framework: a regional 2-year audit of diabetes care in the UK. Diabet Med 27:354–359
Dinneen SF, Gerstein HC (1997) The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus: a systematic overview of the literature. Arch Intern Med 157:1413–1418
Borch-Johnsen K, Feldt-Rasmussen B, Strandgaard S, Schroll M, Jensen JS (1999) Urinary albumin excretion: an independent predictor of ischemic heart disease. Arterioscler Thromb Vasc Biol 19:1992–1997
Schmieder RE, Mann JF, Schumacher H, on behalf of the ONTARGET Investigators et al (2011) Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Am Soc Nephrol 22:1353–1364
Araki S, Haneda M, Sugimoto T et al (2005) Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes 54:2983–2987
de Galan BE, Perkovic V, Ninomiya T, ADVANCE Collaborative Group et al (2009) Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 20:883–892
Katayama S, Moriya T, Tanaka S et al for the Japan Diabetes Complications Study Group (2011) Low transition rate from normo- and low microalbuminuria to proteinuria in Japanese type 2 diabetic individuals: the Japan Diabetes Complications Study (JDCS). Diabetologia 54:1025–1031
Acknowledgements
We thank the participants and general practitioners who took part in the study. H. Obata (National Obihiro Hospital, Obihiro, Japan) and M. Ohtaki (Obihiro Kosei General Hospital, Obihiro, Japan) are thanked as members of the endpoint adjudication committee. T. Ninomiya (Kyushu University, Fukuoka, Japan), T. Wada (Kanazawa University, Kanazawa), M. Horio (Osaka University, Osaka, Japan) and K. Iseki (Ryukyu University, Okinawa, Japan) are gratefully acknowledged for critical comments on the manuscript.
Funding
The JDDM cohort is supported by research programme grant funding from the Japan Diabetes Society.
Contribution statement
HY, KK, KH, MO and KS were principal investigators and were responsible for the study conception and design in the JDDM cohort. HY, SA, MH, HS, HM and AK drafted the manuscript. HY, SA, MM and HS conducted the principal statistical analysis. KK, KH, MO and KS contributed to the collection and standardisation of the data. HY, SA, MH, MM, HS, HM and AK contributed to the data analysis and interpretation. HY and AS were responsible for data management, computing and data linkage. All authors contributed to the writing of this paper and approved the final version.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
A list of the Japan Diabetes Clinical Data Management Study Group Investigators is shown in the Appendix.
Appendix
Appendix
List of the Japan Diabetes Clinical Data Management Study Group Investigators
K. Doi (Doi Internal Medicine Clinic, Uji), Y. Fukumoto (Fukumoto Clinic, Ibusuki), K. Hirao (HEC Science Clinic, Yokohama), K. Iwasaki (Iwasaki Internal Medicine Clinic, Iwakuni), K. Kawai (Kawai Clinic, Tsukuba), M. Kudo (Kudo Internal Medicine Clinic, Aomori), M. Minami (Minami Internal Medicine Clinic, Fukuoka), H. Ogawara (Akasaka Chuo Clinic, Tokyo), M. Oishi (Oishi Internal Medicine Clinic, Kyoto), H. Sugimoto (Sugimoto Clinic, Kitakyusyu), H. Takeda (Takeda Clinic, Isehara), K. Miyazawa (Miyazawa Clinic, Shikaoi), H. Takamura (Takamura Internal Medicine Clinic, Fussa), M. Takai (Takai Internal Medicine Clinic, Kamakura), C. Takahashi (Fuji Koyama Hospital, Oyama), H. Takagi (Toho University, Tokyo), H. Yokoyama (Jiyugaoka Medical Clinic, Int. Med., Obihiro), T. Wada (Toyo Kohan Clinic, Kudamatsu).
Rights and permissions
About this article
Cite this article
Yokoyama, H., Araki, S., Haneda, M. et al. Chronic kidney disease categories and renal–cardiovascular outcomes in type 2 diabetes without prevalent cardiovascular disease: a prospective cohort study (JDDM25). Diabetologia 55, 1911–1918 (2012). https://doi.org/10.1007/s00125-012-2536-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2536-y