Abstract
Aims/hypothesis
Sirtuin 1 (SIRT1) is a longevity-associated protein, which regulates energy metabolism and lifespan in response to nutrient deprivation. It has been proposed to be a therapeutic target for obesity and metabolic syndrome. We investigated whether α-lipoic acid (ALA) exerts a lipid-lowering effect through regulation of SIRT1 activation and production in C2C12 myotubes.
Methods
ALA-stimulated AMP-activated protein kinase (AMPK), acetyl-CoA carboxylase (ACC), adipose triacylglycerol lipase (ATGL) and fatty acid synthase (FAS) production, as well as intracellular triacylglycerol accumulation and fatty acid β-oxidation were analysed in the absence or presence of a SIRT1 inhibitor (nicotinamide), SIRT1 small interfering (si) RNA and an AMPK inhibitor (compound C) in C2C12 myotubes. Mice with streptozotocin/nicotinamide-induced diabetes and db/db mice fed on a high-fat diet were used to study the ALA-mediated lipid-lowering effects in vivo.
Results
ALA increased the NAD+/NADH ratio to enhance SIRT1 activity and production in C2C12 myotubes. ALA subsequently increased AMPK and ACC phosphorylation, leading to increased palmitate β-oxidation and decreased intracellular triacylglycerol accumulation in C2C12 myotubes. In cells treated with nicotinamide or transfected with SIRT1 siRNA, ALA-mediated AMPK/ACC phosphorylation, intracellular triacylglycerol accumulation and palmitate β-oxidation were reduced, suggesting that SIRT1 is an upstream regulator of AMPK. ALA increased ATGL and suppressed FAS protein production in C2C12 myotubes. Oral administration of ALA in diabetic mice fed on a high-fat diet and db/db mice dramatically reduced the body weight and visceral fat content.
Conclusions/interpretation
ALA activates both SIRT1 and AMPK, which leads to lipid-lowering effects in vitro and in vivo. These findings suggest that ALA may have beneficial effects in the treatment of dyslipidaemia and obesity.
Similar content being viewed by others
Introduction
α-Lipoic acid (ALA) is an endogenous cofactor in many multi-enzyme complexes that catalyse the oxidative decarboxylation of α-keto acids such as pyruvate and α-ketoglutarate. ALA is a powerful antioxidant derived from plants and animal tissues [1]. It has been shown to reduce body weight by suppressing hypothalamic AMP-activated protein kinase (AMPK) [2]. It increases insulin sensitivity and skeletal muscle fatty acid oxidation by activating AMPK in diabetes-prone Otsuka Long Tokushima fatty rats [3, 4]. It also increases glucose uptake in 3T3-L1 adipocytes [5], skeletal muscle cells [6] and skeletal muscles of ob/ob mice [7]. Treatment with ALA ameliorates severe hypertriacylglycerolaemia by inhibiting triacylglycerol synthesis and VLDL-triacylglycerol secretion in Zucker diabetic fatty rats [8]. Thus, ALA has potent beneficial effects on obesity, type 2 diabetes mellitus and dyslipidaemia.
Sirtuin 1 (SIRT1) is a class III NAD+-dependent histone/protein deacetylase that regulates lipid metabolism and lifespan by deacetylating lysine residues on various transcription factors [9]. It deacetylates the transcription factor of forkhead box O protein (FOXO) [10], p53 [11] and peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) to control metabolic functions [12] and biological processes [13] similar to those observed with energy restriction. Decreased SIRT1 production may have a critical role in the pathogenesis of type 2 diabetes [14] and insulin resistance, which is associated with SIRT1 downregulation of type 2 diabetes [15]. SIRT1 is regulated by NAD+, the concentration of nicotinamide (NA), and the activity of nicotinamide phosphoribosyltransferase (NAMPT). When ALA is taken up by cells, it is converted into dihydrolipoate by mitochondrial lipoamide dehydrogenase or the thioredoxin/thioredoxin reductase system, which couples the conversion of NADH into NAD+ and increases the NAD+/NADH ratio. NA, an inhibitor of SIRT1, can be converted into NAD+ by phosphoribosylation catalysed by NAMPT, and NAMPT is activated by nutrient deprivation, exercise and AMPK activation [16]. Therefore, nutrient deprivation may increase SIRT1 activity by increasing the NAD+/NADH ratio and by decreasing the abundance of NA. However, whether ALA regulates SIRT1 activity or production has not been determined.
AMPK, a fuel-sensing enzyme, exerts effects similar to SIRT1 on cellular energy metabolism and mitochondrial biogenesis [17, 18]. Once activated, AMPK phosphorylates acetyl-CoA carboxylase (ACC), thereby blocking its activity. This in turn decreases malonyl-CoA concentration, leading to enhanced mitochondrial fatty acid β-oxidation. The AMPK signalling pathway is thought to be a natural response to reduce dyslipidaemia and to ameliorate insulin resistance [19]. Therefore, activation of SIRT1/AMPK signalling may offer significant pharmacological benefits in treating dyslipidaemia, obesity and metabolic syndrome. Because of the aforementioned metabolic actions of SIRT and AMPK, we investigated whether ALA regulated lipid metabolism through the SIRT1 and AMPK signalling pathway. We demonstrated that ALA activated and induced SIRT1 production and function as an upstream regulator of AMPK. ALA also enhanced ATGL and suppressed fatty acid synthase (FAS) production, which in turn reduced lipid accumulation in C2C12 cells exposed to high glucose. These data suggest that ALA may exert beneficial effects in treating dyslipidaemia and obesity of the metabolic syndrome.
Methods
Materials
DMEM, FCS, glutamine, gentamicin, penicillin and streptomycin were purchased from Life Technologies (Gaithersburg, MD, USA). Rabbit polyclonal antibodies to FAS, acetyl-lysine and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were from Gentex (Irvine, CA, USA). Antibodies to phospho-AMPK, AMPK, ATGL, phospho-ACC and ACC were obtained from Cell Signaling Technology (Danvers, MA, USA). Rabbit polyclonal anti-SIRT1, mouse polyclonal anti- liver kinase B1 (LKB-1) and horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). 5-Aminoimidazole-4-carboxamide riboside (AICAR), compound C and NA were purchased from Calbiochem-Novabiochem (San Diego, CA, USA). HRP-conjugated anti-rabbit IgG was purchased from Bio-Rad (Hercules, CA, USA). SIRT1 assay kits were from BIOMOL (Plymouth Meeting, PA, USA).
Culture of C2C12 myotubes and preparation of cell lysates
C2C12 skeletal muscle cells were cultured in DMEM supplemented with 10% heat-inactivated FCS and penicillin (100 U/ml)/streptomycin (100 mg/ml). After reaching confluence, C2C12 cells were differentiated to myotubes by the addition of 2% horse serum for 96 h as described by Lagouge et al [20], and these myotubes were treated with various concentrations of the indicated agents and incubated for the indicated periods in a 5% CO2 humidified incubator at 37 °C. Cells were scraped off using a rubber policeman to obtain cell lysates for the experiments.
Immunoprecipitation and western blotting
Proteins from cell lysates were separated by SDS-PAGE and transferred to poly(vinylidene difluoride) membranes for immunoblotting. Membranes were blocked with blocking solution containing 3% BSA and 0.1% Tween 20 in PBS for 1 h at room temperature followed by incubation with the primary and secondary antibodies. For immunoprecipitation, the agarose beads were conjugated with antibody to LKB-1. Protein (500 μg) from cultured cells was incubated with cross-linked LKB-1 beads overnight, and the immunoprecipitates were boiled with sample loading buffer containing 0.5 mol/l TRIS/HCl (pH 6.8), 4.4% (wt/vol.) SDS, 20% (vol./vol.) glycerol, 2% (vol./vol.) 2-mercaptoethanol and bromophenol blue in distilled/deionised water for 5 min before SDS-PAGE. Immunodetection was performed using a LumiGLO chemiluminescence kit (Amersham International, Amersham, UK). Levels of phosphorylation and abundance were quantified by scanning densitometry using a model GS-700 imaging densitometer (Bio-Rad), normalised to levels of total protein.
Transfection of SIRT1-specific small interfering (si) RNA
C2C12 cells were seeded at 5 × 105 cells per 6 cm plate and allowed to adhere overnight. SIRT1-specific siRNA was transfected into cells using lipofectamine (Invitrogen, Grand Island, NY, USA) after 48 h of differentiation. After 48 h, transfected cells were treated with ALA (300 μmol/l) for 24 h. Cell lysates were collected in parallel for the western blot analysis.
Measurement of SIRT1 activity
SIRT1 activity was assayed by a Fluor-de-Lys fluorescence assay kit (BIOMOL) according to the manufacturer’s instructions. Briefly, the assay was performed by incubating recombinant human SIRT1 protein and substrates, including a fluorogenic acetylated Lys382 p53 peptide (50 μmol/l) and NAD+ (100 μmol/l) at 37 °C for 30 min according to the manufacturer’s instructions. The fluorescence intensity was measured using a Fluoroskan Ascent microplate fluorimeter (Thermo Electron, Milford, MA, USA). Negative controls included ‘no enzyme’ and ‘time zero’ controls, in which Developer II solution plus 2 mmol/l NA was added before mixing of the substrates with or without the SIRT1 enzyme. SIRT1 activity was calculated with the corrected arbitrary fluorescence units of the tested compounds to the ‘no enzyme’ control and expressed as fluorescent units relative to the control. To rule out ALA possession of autofluorescence or interference of NA itself with the fluorescent signal, the Developer II solution was incubated with the Fluor-de-Lys deacetylated standard or the tested compounds in the absence of SIRT1 enzyme or substrates. The deacetylated standard dose-dependently increased the fluorescent rate, whereas the tested compounds did not alter the fluorescent intensity, indicating that the change in p53 deacetylation caused by these compounds depends on the specific SIRT1 activity.
Palmitate β-oxidation
After differentiation, cells were resuspended in medium supplemented with [9,10-3H]palmitate in a mixture of the palmitate and 10% BSA at a 1: 2 volume ratio. In total, 3.3 μl [9,10-3H]palmitate and 6.7 μl BSA were used per ml of cell culture medium. Each sample used 0.5 × 106 cells in 1 ml medium supplemented with the [9,10-3H]palmitate/BSA mixture and cultured for 24 h in 24-well plates. After 24 h, the supernatant fraction was applied to an ion-exchange column (Dowex 1X8-200; Sigma, St Louis, MO, USA), and 3H-labelled water was recovered by elution with 2.5 ml water. A 0.75 ml aliquot was then used for scintillation counting.
Measurement of intracellular triacylglycerol content
Intracellular triacylglycerol was assayed using a colorimetric assay (GPO-Trinder Reagent A; Sigma) [21]. Briefly, 30 μl triacylglycerol standard or supernatant fraction was added to a 96-well flat-bottom polystyrene plate, and 300 μl triacylglycerol reagent was then added to the microplate. The plate was incubated for 5 min, and the absorbance was determined at 520 nm with a Gen5 data collector (BioTek Instruments, Highland Park, Winooski, VT, USA). Intracellular triacylglycerol levels were normalised to total protein and expressed as μg lipid/mg protein.
NAD+/NADH ratio measurement
The NAD+/NADH ratio was measured from whole-cell extracts of C2C12 myotubes or liver using the Biovision NAD+/NADH quantification kit (Biovision, San Francisco, CA, USA). To detect total NAD+ (NADt [NAD+ + NADH]), 50 μl extracted sample (cell or tissue) was transferred to labelled 96-well plates. Into each well of the NADH standard, 100 μl of the NAD+ Cycling Mix was added at room temperature for 5 min to convert NAD+ into NADH. Then 10 μl NADH developer was added to each well for 1–4 h. The absorbance was determined at 450 nm with a Biotex Microplate Spectrophotometer. To detect NADH, NAD+ needs to be decomposed before the reaction by heating at 60 °C for 30 min. The sample readings were applied to the NADH standard curve, and the amounts of NADt and NADH were determined using the equation. At the same time, the NAD+/NADH ratio was calculated as: (NADt − NADH)/NADH. These readings were normalised to the cell number (cell sample) and protein concentration (tissue sample).
Animals
The db/db mice on a C57BL/6 background (male, 14 weeks old, 50 g) were gifts from the Development Center for Biotechnology of Taiwan. The animals were given free access to water and were fed on a standard diet. ALA (200 mg/kg) or vehicle was administered orally in the afternoon (14:00–14:30 hours). The serum biochemical profiles, including triacylglycerol, cholesterol, insulin, HDL-cholesterol, LDL-cholesterol, aspartate aminotransferase and alanine aminotransferase, were determined with a Biochem-Immuno autoanalyser (Brea, CA, USA). Whole blood levels of biochemical variables, including HbA1c and glucose, were also measured with a Biochem-Immuno autoanalyser. The quality controls, calibrations and determining procedures were carried out according to the suppliers’ instructions. The glucose tolerance test and histopathological examinations were performed on day 90.
Streptozotocin/NA-induced diabetes mouse model
Male C57BL/6J (6 weeks old) mice were obtained from the Taiwan National Laboratory Animal Center. Streptozotocin (STZ, 100 mg/kg) was administered to C57BL/6J mice with NA (240 mg/kg), twice with an interval of 2 days, and then these mice were fed on a high-fat diet for 4 weeks to induce type 2 diabetes [22]. Mice were randomly separated into three groups for treatment with 0, 50 or 200 mg/kg by oral administration. Plasma glucose concentration and body weight were measured on days 0, 7, 14 and 28. Biochemical measurements, glucose tolerance and pathological examinations were performed on day 30.
Histochemical analysis
Liver and muscle were fixed and embedded in tissue-freezing medium (Leica Microsystems, Wetzlar, Germany) and stored at −80 °C. The frozen tissue was cut into 7 μm-thick sections and placed on glass slides. The tissue sections were stained with haematoxylin and eosin, Oil Red or Sudan III. Oil Red staining and Sudan III staining were counterstained with haematoxylin to visualise lipid droplets.
Statistical analysis
All data are expressed as the mean±SEM, and statistical significance (p < 0.05) between experimental groups was determined by a single-factor ANOVA for multiple groups or unpaired t test for two groups.
Results
ALA increases SIRT1 activity and production by modulating the NAD+/NADH ratio
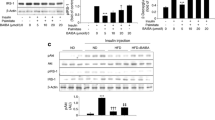
Recent studies have suggested that SIRT1, a mammalian orthologue of Sirt2, is closely associated with lipid metabolism and obesity [15, 23, 24]. Resveratrol, a well-known antioxidant, has been shown to stimulate SIRT1 activity [25]. ALA is also a naturally occurring antioxidant with potent reactive oxygen species-scavenging activity. To test whether ALA increases SIRT1 deacetylase activity, we first determined ALA-stimulated deacetylation of a fluorogenic acetylated Lys382 p53 peptide, and the result showed that incubation with ALA (30 μmol/l) significantly increased SIRT1 activity (Fig. 1a). Since SIRT1 is an NAD+-dependent enzyme that acts as a metabolic sensor of NAD+, we next measured the NAD+/NADH ratio in C2C12 myotubes. Treatment of cells with ALA for 24 h significantly increased NAD+ and the NAD+/NADH ratio in C2C12 myotubes (Fig. 1b and d). As an increase in the NAD+/NADH ratio is known to induce SIRT1 production, we examined the protein level of SIRT1 in C2C12 myotubes. As shown in Fig. 1e, treatment with ALA caused an increase in SIRT1 protein levels in C2C12 myotubes.
ALA increases SIRT1 activity and production by modulating the NAD+/NADH ratio. a A Fluor-de-Lys fluorescence assay was performed using a synthetic acetylated Lys382 p53 peptide and NAD+ as the substrate. SIRT1 activity is expressed as arbitrary fluorescence units relative to the control. Data are expressed as the mean±SEM (n = 3). *p < 0.05 compared with control. Intracellular levels of NAD+ (b) and NADH (c), and the NAD+/NADH ratio (d) in C2C12 myotubes treated with ALA (300 and 1,000 μmol/l) for 24 h. e Cells were incubated with 300 μmol/l ALA for the indicated time periods (1, 2, 6, 12, and 24 h). The cell lysate was immunodetected with a SIRT1-specific antibody
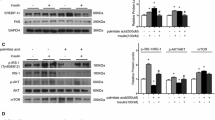
ALA stimulates AMPK phosphorylation and LKB-1 deacetylation
AMPK exerts effects similar to SIRT1 on cellular energy metabolism and mitochondrial biogenesis. To investigate whether ALA stimulates the AMPK signalling pathway, we examined the effects of ALA on phosphorylation of AMPK and ACC, a downstream target of AMPK. Figure 2 shows that ALA increased phosphorylation of AMPK and ACC in C2C12 myotube cells in a concentration-dependent manner (Fig. 2a–c). To determine whether the activation of AMPK promoted fatty acid β-oxidation, we examined the effect of ALA on palmitate β-oxidation. ALA (30–1,000 μmol/l, 24 h) increased the rate of β-oxidation in a concentration-dependent manner (Fig. 2d). LKB-1, an upstream regulator of AMPK, has been shown to be regulated by SIRT1-dependent deacetylation [26]. To determine whether SIRT1 regulated LKB-1 deacetylation, the acetylation level of LKB-1 was measured by immunoprecipitation of LKB-1 followed by immunobloting with antibodies to acetyl-lysine. As shown in Fig. 2e, treatment with ALA or resveratrol (a well-known SIRT1 activator) reduced the acetylation level of LKB-1, where the levels of acetylation were normalised to protein input as shown in Fig. 2f. Both resveratrol and ALA significantly reduced LKB-1 acetylation. These data support the notion that SIRT1 may regulate the AMPK signalling pathway through LKB-1 deacetylation [27].
ALA stimulates AMPK phosphorylation and LKB-1 deacetylation. a C2C12 myotubes were treated with different concentrations of ALA and AICAR (A, 1 mmol/l) for 1 h. Phosphorylation of AMPK and ACC was analysed by immunoblotting with an AMPK-phosphorylated antibody at Thr172 and an ACC-phosphorylated antibody at Ser79. b and c Ratios of pAMPK to AMPK and pACC to ACC, respectively, were quantified in three independent experiments. Data are expressed as the mean±SEM (n = 3). *p < 0.05 compared with the untreated control. d C2C12 myotubes were treated with different concentrations of ALA for 24 h, at 37 °C. [3H]Palmitate was added, and fatty acid β-oxidation was assayed as described in the Methods section. Data are expressed as the mean±SEM (n = 3). *p < 0.05 compared with the control (0 μmol/l). e C2C12 myotubes cells treated with ALA (300 μmol/l) for 3 h. Protein lysates were immunoprecipitated (IP) with antibodies to LKB-1, and acetylation level was detected by imunoblotting (IB) with antibodies to acetyl-lysine (Ac-lys). RSV, resveratrol. f The acetylation levels were normalised to protein input. Data are expressed as the mean±SEM (n = 3). *p < 0.05 compared with the control. Black bar, acetylation; white bar, IgG. Con, control; RSV, resveratrol
ALA-increased [9,10-3H]palmitate β-oxidation is mediated through SIRT1/AMPK signalling
To test whether SIRT1 acts as an upstream regulator of AMPK, we investigated the effects of pharmacological inhibitors of SIRT1 (NA) and AMPK (compound C) on phosphorylation of AMPK and ACC. As shown in Fig. 3a–c, ALA increased AMPK and ACC phosphorylation, and these effects were blocked by NA, suggesting that SIRT1 acts upstream of AMPK. We next examined the effects of these inhibitors on fatty acid β-oxidation in C2C12 cells. ALA increased [9,10-3H]palmitate β-oxidation in C2C12 cells, and this effect was inhibited by pretreatment with NA or compound C (Fig. 3d). To further confirm the role of SIRT1 in regulating AMPK activation, SIRT1-specific siRNAs were transfected to knockdown SIRT1 production. Knockdown of SIRT1 significantly decreased ALA-stimulated AMPK and ACC phosphorylation (Fig. 3e) along with a decrease in fatty acid β-oxidation (Fig. 3f). Taken together, our data suggest that ALA stimulates AMPK signalling and increases fatty acid β-oxidation in a SIRT-1-dependent manner.
ALA-increased [9,10-3H]palmitate β-oxidation is mediated through (SIRT1)/AMPK signalling. a C2C12 myotubes were pretreated with NA (1 nmol/l) for 24 h and compound C (20 μmol/l) for 30 min before the addition of ALA (300 μmol/l) for 1 h. b and c Ratios of pAMPK to AMPK and pACC to ACC, respectively, were quantified in three independent experiments per condition. Data are expressed as the mean±SEM (n = 3). *p < 0.05 compared with the control (0 μmol/l). d C2C12 myotubes were pretreated with compound C (20 μmol/l) or NA (1 nmol/l) for 24 h before the addition of ALA (300 μmol/l) for 24 h. [3H]Palmitate was added, and fatty acid β-oxidation was assayed. e C2C12 cells were transfected with SIRT1 siRNA for 72 h and then stimulated with ALA for 30 min. Cell lysates were analysed by immunoblotting using antibodies specific for SIRT1, pAMPK, pACC, AMPK and GAPDH. These results represent one of three independent experiments. f C2C12 cells were transfected with SIRT1 siRNA for 72 h. Fatty acid β-oxidation was examined with [3H]palmitate in C2C12 myotubes. All above data are expressed as the mean±SEM (n = 3). *p < 0.05 compared with the control (0 μmol/l) and †p < 0.05 compared with ALA treated alone group. AICAR (1 mmol/l) was used as a positive control
ALA regulates lipid metabolism and lowers high-glucose-induced cellular triacylglycerol accumulation
To determine whether ALA is involved in lipid metabolism, we examined the expression of lipid-related proteins in C2C12 myotubes. The production of FAS was suppressed by treatment of C2C12 myotubes with ALA (30–1,000 μmol/l) for 24 h. In contrast, treatment with ALA increased the production of ATGL (Fig. 4a). ATGL is a newly discovered lipase, which catalyses the hydrolysis of the first ester bond of stored triacylglycerol [28]. Incubation of cells in high-glucose medium is known to increase lipogenic gene expression and lipid accumulation. Consistently, intracellular triacylglycerol accumulation was increased in the high-glucose condition, and the increase in accumulation was blocked by pretreatment with ALA (100–1,000 μmol/l). To delineate the roles of SIRT1 and AMPK in lipid metabolism, cells were pretreated with a SIRT1 inhibitor (NA) or an AMPK inhibitor (compound C), and their effects on FAS and ATGL production were examined. As shown in Fig. 4c, the effects of ALA on FAS and ATGL production were reversed by the pharmacological inhibitor for AMPK or SIRT1, suggesting that the regulation of these enzymes is mediated through SIRT1- and AMPK-dependent pathways. Consistently, knockdown of SIRT1 by siRNA reversed the regulation by ALA of FAS and ATGL production (Fig. 4d). Inhibition of SIRT1 and AMPK or knockdown of SIRT1 also attenuated ALA-induced lipid reduction under high-glucose conditions (Fig. 4e, f). These data suggest that ALA protected against high-glucose-induced triacylglycerol accumulation by regulating lipid-related proteins.
ALA regulates metabolism and lowers high-glucose-induced cellular triacylglycerol accumulation. a C2C12 myotubes were treated with different concentrations of ALA and AICAR (A, 1 mmol/l) for 24 h. Whole-cell lysate protein was examined by western blotting for FAS and ATGL. b C2C12 myotubes were incubated for 24 h without or with increasing concentrations of ALA in the absence or presence of 30 nmol/l d-glucose (high glucose). Intracellular triacylglycerol contents were measured and are expressed as μg of lipid/mg of protein (mean±SEM, n = 3) as described in the Methods section. *p < 0.05 vs normal glucose; †p < 0.05 vs high glucose alone; ‡p = 0.05. c and d Cells were pretreated with NA or compound C for 24 h and then incubated with ALA for another 24 h. The production of SIRT1, FAS and ATGL was detected by western blotting. Intracellular triacylglycerol was also measured (mean±SEM, n = 3; *p < 0.05 vs ALA treatment under high-glucose treatment). e and f Knockdown of SIRT1 by siRNA downregulates SIRT1 production and FAS, ATGL and intracellular triacylglycerol levels in C2C12 myotubes treated with ALA. *p < 0.05 vs ALA treatment under high-glucose treatment
ALA ameliorates the metabolic function of mice with STZ/NA-induced diabetes
To investigate the effects of ALA on lipid metabolism in vivo, C57BL/6J mice were administered STZ (100 mg/kg) and NA (240 mg/kg), twice with an interval of 2 days. These mice were then fed on a high-fat diet for 4 weeks to induce type 2 diabetes mellitus [22]. ALA was administered daily orally (50 and 200 mg/kg) for 1 month. Administration of ALA markedly reduced the mass of visceral fat in mice with STZ/NA-induced diabetes (Fig. 5a). The fat mass was significantly reduced by ALA treatment. The reductions in the mass of fat cells in 50 and 200 mg/kg-treated mice were 14.36 and 52.58%, respectively (Fig. 5c). The vehicle-treated mice showed normal body weight during the experiment period, whereas ALA treatment at 200 mg/kg caused a dramatic loss (~11%) of body weight (Fig. 5b), and did not improve glucose tolerance in diabetic mice (electronic supplementary material [ESM] Fig. 1). Although the liver weight of ALA-treated mice showed no difference, the liver function index of alanine aminotransferase of ALA-treated mice (18.00 ± 2.16) was obviously improved compared with that of vehicle-treated mice (54.60 ± 7.23; Table 1). Plasma glucose and triacylglycerol levels were lower in the ALA-treated group at a high dosage (Table 1). These data suggest that the metabolic dysfunction in STZ/NA-induced diabetic mice was alleviated with ALA treatment.
ALA ameliorates the metabolic function in mice with STZ- and NA-induced diabetes. a Gross appearance of the whole body and abdomen of diabetic mice treated with vehicle (50% alcohol, control, left) or ALA (middle, 50 mg kg−1 day−1 [low]; right, 200 mg kg−1 day−1 [high]). b Changes in body weight with or without ALA. Diabetic mice were treated with the vehicle (white circle; n = 7) or ALA (upside down black triangle, 50 mg/kg, n = 7; white triangle, 200 mg/kg, n = 6) and the normal group (black circle, n = 5). *p < 0.05 compared with the vehicle group. c The mass of visceral fat was measured. *p < 0.05 compared with the normal group and †p < 0.05 compared with diabetic vehicle group. d Western blot analysis of lipid-related protein production in diabetic mice liver with or without ALA. e Intracellular levels of NAD+ (e), NADH (f) and the NAD+/NADH ratio (g) in the liver of mice treated with high (200 mg/kg) or low (50 mg/kg) doses of ALA. Results are the mean±SEM. *p < 0.05
ALA regulates lipid-related proteins, the NAD+/NADH ratio, and lipid accumulation in STZ/NA-induced diabetic mice
To further confirm the changes in FAS and ATGL in vivo, we examined the levels of ATGL, FAS and phospho-AMPK in the liver of STZ/NA-induced diabetic mice. ALA treatment decreased FAS production and increased ATGL and phospho-AMPK levels (Fig. 5d). The NAD+ level was increased from 278 to 575 pmol/μg protein, and the NAD+/NADH ratio was increased by more than 50% (Fig. 5e–g).
Effects of ALA treatment in db/db mice
Because STZ/NA-induced diabetic mice may have unphysiological metabolic conditions, db/db mice were used to confirm our observations. ALA was orally administered to 14-week-old db/db mice for 3 months. ALA administration resulted in a 13% reduction in body weight compared with the control group (Fig. 6a, b). The gonadal fat tissues were also significantly but slightly decreased in ALA-treated mice (Fig. 6c). The visceral fat content in db/db mice was significantly reduced in those treated with ALA (Fig. 6d). Serum triacylglycerol, cholesterol and LDL levels were significantly lower in the ALA-treated group. Blood HbA1c and glucose levels were not significantly altered by ALA treatment (Table 2). ALA treatment did not alter the glucose tolerance in db/db mice (ESM Fig. 2). We next assessed the effects of ALA on lipid-related gene expression in isolated liver and skeletal muscle. Consistent with our in vitro data, the production of ATGL, SIRT1 and phospho-AMPK was upregulated by ALA treatment, whereas FAS was suppressed (Fig. 6e). The liver and muscle sections were stained with Oil Red-O and Sudan III to detect lipid. As shown in Fig. 6f,g, fewer lipid droplets were observed in ALA-treated mice than in the untreated group.
ALA improves lipid accumulation in db/db mice. a Gross appearance of the whole body and abdomen of db/db mice treated with vehicle (50% alcohol, control, left) or ALA (right, 200 mg kg−1 day−1). b Changes in body weight with or without ALA. The db/db mice were treated with the vehicle (white circle; n = 4) or ALA (black circle, 200 mg/kg, n = 4). *p < 0.05 compared with the vehicle group. The gonadal (c) and visceral (d) fat pads were isolated from control and ALA-treated mice. e Western blot analysis of lipid-related protein production in db/db mice liver and muscle with or without ALA. f, g Histopathological sections of muscle and liver from ALA-treated and -untreated db/db mice were stained with Oil Red-O and Sudan III to detect the content of lipid droplets. H&E, haematoxylin and eosin
Discussion
Obesity, dyslipidaemia and metabolic syndrome are increasingly prevalent due to excess energy intake and nutrient availability. Energy restriction and exercise may prevent metabolic disorders, but are seldom successful. Thus there is an unmet medical need to develop therapeutic agents that mimic energy restriction and exercise to combat metabolic disorders. SIRT1 is thought to play a pivotal role in metabolic adaptation to energy restriction, and is proposed as a potential target for treating metabolic disorders. This study provides the first evidence that SIRT1 is a target of ALA. ALA activated SIRT1 in a cell-free system, and ALA also increased the NAD+/NADH ratio and upregulated SIRT1 in C2C12 myotubes. Furthermore, we demonstrated that ALA enhanced fatty acid β-oxidation, increased ATGL production, and decreased FAS production through the SIRT1/AMPK signalling pathway.
ALA is a strong antioxidant which can be reduced to dihydrolipoase by either mitochondrial lipoamide dehydrogenase or the thioredoxin/thioredoxin reductase system to increase the NAD+/NADH ratio. The ability of ALA to increase NAD+ and the NAD+/NADH ratio may explain, at least in part, how ALA treatment increases SIRT1 activity. On the other hand, activation of AMPK was linked to SIRT1 protein production in skeletal muscle cells [29]. As ALA activates AMPK, another possibility is that ALA induces SIRT1 production through AMPK activation. ALA-stimulated AMPK phosphorylation was inhibited by the SIRT1 inhibitor, suggesting that SIRT1 acts as an upstream activator of AMPK. These observations were further confirmed by the knockdown of SIRT1, which also reduced AMPK phosphorylation in C2C12 myotubes. Our results agree with those of Hou et al [30], who showed that polyphenols mediate lipid-lowering effects through the SIRT1/LKB1/AMPK signalling pathway in HepG2 cells. Our results are in line with those of Lan et al [31], who demonstrated that SIRT1 leads to deacetylation of a lysine residue on LKB-1 to activate AMPK. However, these results differ from the findings of Canto et al [32], who showed that AMPK acted upstream of SIRT1 by modulating NAD+ metabolism. AMPK has been shown to increase SIRT1 activity by regulating NAMPT, an NAD+-biosynthetic enzyme [33]. On the other hand, SIRT1 was upregulated by the p53/FOXO3a complex [14], and AMPK has been shown to regulate FOXO1 activity through phosphorylation [34]. Although AMPK and SIRT1 can activate each other in different orders, the two molecules have similar effects on energy metabolism. These findings support the notion that cellular metabolism is regulated in a concerted manner.
We demonstrated that oral administration of ALA decreased body weight and visceral fat mass in diabetic mice fed on a high-fat diet and in db/db mice. The lipid-lowering effect of ALA was associated with increased ATGL and suppressed FAS protein production in liver and skeletal muscle of both STZ/NA-induced and db/db diabetic mice. These responses were attenuated by inhibition of SIRT1 or AMPK, suggesting that these lipid metabolism-related proteins were regulated through a SIRT1/AMPK-dependent pathway. These results agree with those reported by Hou et al, who showed that activation of SIRT1 by resveratrol exerted a lipid-lowering effect though AMPK [30]. These data also agree with those of Gaidhu et al [35], who reported that AICAR induces AMPK activation, which promotes energy dissipation through ATGL induction. On the other hand, it is well known that AMPK phosphorylates and inhibits ACC activity, which in turn increases fatty acid oxidation. Inhibition of FAS, a lipogenic enzyme, is another important consequence of AMPK activation by ALA. These findings are in accordance with results of previous studies showing that FAS gene expression was abrogated by treatment with AICAR in hepatocytes [36]. These results define a novel mechanism by which ALA has a lipid-lowering effect by activating SIRT1 and AMPK and by regulating lipid-related proteins of ACC, ATGL and FAS. These observations are consistent with results showing amelioration of insulin sensitivity and triacylglycerol clearance by ALA in skeletal muscle and liver [8]. However, the impaired glucose tolerance was not improved by ALA treatment in either STZ/NA-induced or db/db diabetic mice (ESM Figs 1 and 2). Although HbA1c was slightly reduced, the differences between treated and untreated groups were not statistically significant (Table 2). These data suggest that treatment with ALA alone may have limitations for exerting an anti-glycaemic effect.
Taken together, ALA exerts an anti-obesity effect by activating the SIRT1/AMPK signalling pathway. This study demonstrates novel pharmacological actions of ALA, including activation of SIRT1 in muscle cells and regulation of lipid-related proteins. ALA not only enhances fatty acid oxidation but also promotes a lipid-lowering effect by regulating FAS and ATGL expression. Oral administration of ALA decreased body weight and the visceral fat content of both db/db and STZ/NA-induced diabetic mice.
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- AICAR:
-
5-Aminoimidazole-4-carboxamide riboside
- ALA:
-
α-Lipoic acid
- AMPK:
-
AMP-activated protein kinase
- ATGL:
-
Adipose triacylglycerol lipase
- FAS:
-
Fatty acid synthase
- FOXO:
-
Forkhead box O
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- HRP:
-
Horseradish peroxidase
- LKB-1:
-
Liver kinase B1
- NA:
-
Nicotinamide
- NAMPT:
-
Nicotinamide phosphoribosyltransferase
- siRNA:
-
Small interfering RNA
- SIRT-1:
-
Sirtuin 1
- STZ:
-
Streptozotocin
References
Reed LJ (2001) A trail of research from lipoic acid to alpha-keto acid dehydrogenase complexes. J Biol Chem 276:38329–38336
Kim MS, Park JY, Namkoong C et al (2004) Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 10:727–733
Evans JL, Goldfine ID (2000) Alpha-lipoic acid: a multifunctional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. Diabetes Technol Ther 2:401–413
Lee DH, Park DB, Lee YK et al (2005) The effects of thiazolidinedione treatment on the regulations of aquaglyceroporins and glycerol kinase in OLETF rats. Metabolism 54:1282–1289
Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A (2000) Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by alpha-lipoic acid in 3T3-L1 adipocytes. Diabetologia 43:294–303
Konrad T, Vicini P, Kusterer K et al (1999) Alpha-lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes Care 22:280–287
Eason RC, Archer HE, Akhtar S, Bailey CJ (2002) Lipoic acid increases glucose uptake by skeletal muscles of obese-diabetic ob/ob mice. Diabetes Obes Metab 4:29–35
Butler JA, Hagen TM, Moreau R (2009) Lipoic acid improves hypertriglyceridemia by stimulating triacylglycerol clearance and downregulating liver triacylglycerol secretion. Arch Biochem Biophys 485:63–71
Guarente L (2006) Sirtuins as potential targets for metabolic syndrome. Nature 444:868–874
Brunet A, Sweeney LB, Sturgill JF et al (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015
Langley E, Pearson M, Faretta M et al (2002) Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J 21:2383–2396
Gerhart-Hines Z, Rodgers JT, Bare O et al (2007) Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26:1913–1923
Funk JA, Odejinmi S, Schnellmann RG et al (2010) SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther 333:593–601
Rutanen J, Yaluri N, Modi S et al (2010) SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes 59:829–835
de Kreutzenberg SV, Ceolotto G, Papparella I et al (2010) Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes 59:1006–1015
Vinciguerra M, Fulco M, Ladurner A, Sartorelli V, Rosenthal N (2010) SirT1 in muscle physiology and disease: lessons from mouse models. Dis Model Mech 3:298–303
Winder WW, Holmes BF (2000) Insulin stimulation of glucose uptake fails to decrease palmitate oxidation in muscle if AMPK is activated. J Appl Physiol 89:2430–2437
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 104:12017–12022
Ouadda AB, Levy E, Ziv E et al (2009) Increased hepatic lipogenesis in insulin resistance and Type 2 diabetes is associated with AMPK signalling pathway up-regulation in Psammomys obesus. Biosci Rep 29:283–292
Lagouge M, Argmann C, Gerhart-Hines Z et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell 127:1109–1122
Wang YX, Lee CH, Tiep S et al (2003) Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 113:159–170
Nakamura T, Terajima T, Ogata T et al (2006) Establishment and pathophysiological characterization of type 2 diabetic mouse model produced by streptozotocin and nicotinamide. Biol Pharm Bull 29:1167–1174
Kume S, Uzu T, Kashiwagi A, Koya D (2010) SIRT1, a calorie restriction mimetic, in a new therapeutic approach for type 2 diabetes mellitus and diabetic vascular complications. Endocr Metab Immune Disord Drug Targets 10:16–24
Lomb DJ, Laurent G, Haigis MC (2009) Sirtuins regulate key aspects of lipid metabolism. Biochim Biophys Acta 1804:1652–1657
Howitz KT, Bitterman KJ, Cohen HY et al (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425:191–196
Alessi DR, Sakamoto K, Bayascas JR (2006) LKB1-dependent signaling pathways. Annu Rev Biochem 75:137–163
Zheng Z, Chen H, Li J et al (2012) Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes 61:217–228
Zimmermann R, Strauss JG, Haemmerle G et al (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386
Suwa M, Nakano H, Radak Z, Kumagai S (2010) Short-term adenosine monophosphate-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside treatment increases the sirtuin 1 protein expression in skeletal muscle. Metabolism 60:393–403
Hou X, Xu S, Maitland-Toolan KA et al (2008) SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283:20015–20026
Lan F, Cacicedo JM, Ruderman N, Ido Y (2008) SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 283:27628–27635
Canto C, Gerhart-Hines Z, Feige JN et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060
Fulco M, Cen Y, Zhao P et al (2008) Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14:661–673
Nemoto S, Fergusson MM, Finkel T (2004) Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 306:2105–2108
Gaidhu MP, Fediuc S, Anthony NM et al (2009) Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res 50:704–715
Woods A, Azzout-Marniche D, Foretz M et al (2000) Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol 20:6704–6711
Funding
This study was supported by grants NSC 99-3112-B-166-001 and NSC 98-3112-B-038-001 from the National Science Council, Taipei, Taiwan.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
H-ML, S-GW and W-LC contributed to conception and design of the study; W-LC and C-HK contributed to analysis and interpretation of data. W-LC and H-ML drafted the article and S-GW, W-LC and C-HK revised the article for intellectual content. All authors gave final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, WL., Kang, CH., Wang, SG. et al. α-Lipoic acid regulates lipid metabolism through induction of sirtuin 1 (SIRT1) and activation of AMP-activated protein kinase. Diabetologia 55, 1824–1835 (2012). https://doi.org/10.1007/s00125-012-2530-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2530-4