Abstract
Aims/hypothesis
The ability of pancreatic beta cells to proliferate is critical both for normal tissue maintenance and in conditions where there is an increased demand for insulin. Protein kinase B (Akt) plays a major role in promoting proliferation in many cell types, including the insulin-producing beta cells. We have previously reported that mice overexpressing a constitutively active form of Akt (caAkt Tg) show enhanced beta cell proliferation that is associated with increased protein levels of cyclin D1, cyclin D2 and cyclin-dependent kinase inhibitor 1A (p21Cip). In the present study, we sought to assess the mechanisms responsible for augmented p21Cip levels in caAkt Tg mice and test the role of p21Cip in the proliferative responses induced by activation of Akt signalling.
Methods
To gain a greater understanding of the relationship between Akt and p21Cip, we evaluated the mechanisms involved in the modulation of p21Cip by Akt and the in vivo role of reduced p21Cip in proliferative responses induced by Akt.
Results
Our experiments showed that Akt signalling regulates p21 Cip transcription and protein stability. caAkt Tg /p21 Cip+/− mice exhibited fasting and fed hypoglycaemia as well as hyperinsulinaemia when compared with caAkt Tg mice. Glucose tolerance tests revealed improved glucose tolerance in caAkt Tg /p21 Cip+/− mice compared with caAkt Tg. These changes resulted from increased proliferation, survival and beta cell mass in caAkt Tg /p21 Cip+/− compared with caAkt Tg mice.
Conclusions/interpretation
Our data indicate that increased p21Cip levels in caAkt Tg mice act as a compensatory brake, protecting beta cells from unrestrained proliferation. These studies imply that p21Cip could play important roles in the adaptive responses of beta cells to proliferate in conditions such as in insulin resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The capacity of the pancreatic beta cells to proliferate in response to insulin resistance is critical for glucose homeostasis and for preventing the progression to type 2 diabetes. Proliferation of mature beta cells is one of the components responsible for maintenance of beta cell mass in adult life [1]. Although it is widely accepted that reduction in functional beta cell mass is key in the pathogenesis of both type 1 and type 2 diabetes, there is little understanding of the mechanisms of how beta cells enter the cell cycle and progress to cell expansion. Many growth factors, including insulin and IGF-1, have been demonstrated to regulate beta cell mass [2–5]. The mechanisms downstream of these growth factor receptors are not fully mapped out, but it is widely accepted that the IRS2/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway plays a major role in the regulation of beta cell mass [6–10]. Indeed, numerous in vivo and in vitro studies point to the serine/threonine Akt kinase as a critical regulator of both proliferation and survival of beta cells [11–14]. We have previously demonstrated that Akt induces beta cell proliferation by promoting cyclin-dependent kinase 4 (CDK4) activity [15]. While there is some knowledge about the signalling pathways regulating beta cell proliferation, the downstream signalling pathways linking Akt to activation of CDK4 activity and beta cell G1–S transition are not completely understood.
Recent work from several laboratories has shown that CDK4 and cyclins D1 and D2 are critical regulators of proliferation and maintenance of beta cell mass after birth [16–19]. The CDK4-6/cyclin D complex is inhibited by the INK4 family (p16INK4a, p15INK5b, p18INK4c and p19INK4d) of cell cycle inhibitors. p21Cip belongs to the Cip/Kip family of inhibitors (p21Cip, p27Kip1 and p57Kip2) and primarily inhibits CDK2. In addition to their cell cycle inhibitory function, p27Kip and p21Cip are also positive regulators of cyclin D/CDK4 complexes by promoting their assembly, stabilisation and activation in a concentration-dependent manner [20–23]. Understandably, repression of p21Cip decreases formation of the CDK4/CDK6/cyclin D1 complex and results in impaired cell cycle progression in some systems [24, 25]. While the importance of p27 in the regulation of beta cell mass in vivo has been studied, the role of p21Cip in the regulation of the beta cell cycle is not completely understood [26–28]. Experiments in p21Cip-deficient mice have demonstrated that p21Cip is not essential for maintaining beta cell function and cell cycle arrest in vivo [29]. In contrast, transgenic mice overexpressing p21 Cip (also known as Cdkn1a) show decreased beta cell replication and induced hyperglycaemia [30]. Interestingly, increased p21Cip levels have also been demonstrated in proliferative states induced by hepatocyte growth factor, placental lactogen and IGF-I in human islets [31, 32]. Recently, we observed that p21Cip protein levels are increased in beta cells overexpressing Akt [15]. However, the mechanisms involved in the regulation of p21Cip levels by Akt and the role of increased levels of this cell cycle inhibitor in beta cell proliferation are not understood.
In the present study, we set out to determine the mechanisms involved in the regulation of p21Cip levels by Akt and to assess whether this protein plays a role in assembly of the cyclin D/CDK4 complex. The role of p21Cip in cell cycle regulation in vivo was also evaluated by crossing transgenic mice overexpressing a constitutively active Akt in beta cells with mice deficient with p21Cip. These experiments showed that Akt regulates p21Cip levels by increasing its stability and altering its subcellular localisation. Taken together, these in vivo studies suggest that p21Cip acts as a compensatory brake on the mitogenic effects of Akt signalling.
Methods
Generation of caAktTg/p21Cip−/− intercrosses
Mice overexpressing constitutively active Akt1 in beta cells (caAkt Tg) are in a C57BL/6J background and have been described previously [12]. p21 Cip−/− mice were obtained from The Jackson Laboratory (strain B6;129S2 and stock number 003263). Littermate animals used for the study were obtained by generating caAkt Tg /p21 Cip+/− mice first followed by crossing these mice to p21 Cip+/−. This breeding scheme generates the following genotypes: caAkt Tg /p21 Cip−/−, caAkt Tg /p21 Cip+/−, p21 Cip−/−, p21 Cip+/− and p21 Cip+/+. However, we were unable to obtain a sufficient number of caAkt Tg /p21 Cip−/− and the analyses in these studies is limited to the other genotypes. Littermate controls and experimental animals were males on a mixed background. All procedures were performed in accordance with the animal studies committees of the University of Michigan and Washington University.
MIN6 cells and islets isolation
MIN6 cells were stably transfected with a lentivirus containing a constitutively active Akt mutant (MIN6caAkt) and cultured as described previously [33, 34]. MIN6 cells were infected with lentiviral vector containing the constitutively active Akt mutant and selected with G418. The caAkt1 used in this construct lacks the pleckstrin homology domain (Akt∆4-129) and contains an N-terminal Src myristoylation signal (myrAkt∆4-129) along with a haemagglutinin tag and was cultured as described previously [33, 34]. Pancreatic islets from wild-type and caAkt Tg mice were isolated by collagenase treatment as described previously [12]. On the morning following isolation, islets were hand picked, lysed and subjected to immunoprecipitation or immunoblotting analysis.
Cycloheximide treatment of MIN6 cells and islets
MIN6 and MIN6caAkt cells were harvested after incubation in DMEM containing 25 mmol/l glucose, with 10% FBS (Invitrogen, Carlsbad, CA, USA) and cycloheximide (CHX; 12.5 μg/ml) (Sigma, St Louis, MO, USA) for 0, 0.5, 1, 2, 4, 6 or 8 h. The cells were lysed using a lysis buffer (125 mmol/l Tris, pH 7, 2% SDS, 1 mmol/l dithiothreitol) containing a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) and sonicated for 15 s. For islet experiments, islets were cultured in RPMI containing CHX (12.5 μg/ml) for 0 or 1 h. Islet lysates were also processed under the same conditions as described for MIN6 cells. For each sample, 80 μg of total lysate was subjected to immunoblotting as described below using the following antibodies: p21Cip, actin and tubulin.
Western blotting and immunoprecipitation
For immunoblotting, 80 μg total protein was used for probing p21Cip in cell lysates as well as pancreatic islet lysates (∼200 islets). Protein was separated by 15% SDS polyacrylamide gel electrophoresis. Membranes were blocked in 5% non-fat milk for 1 h at room temperature and incubated with primary antibody overnight. The antibodies used were: p21Cip (SX118; BD Biosciences, Franklin Lakes, NJ, USA); phospho-glycogen synthase kinase 3-beta (GSK3β) (Ser9; Cell Signaling, Danvers, MA, USA); CDK4 and CDK2 (c-22 and sc-163, respectively; Santa Cruz Biotechnology, CA, USA). The housekeeping genes actin and tubulin were from Sigma. Cell cycle components were assessed using antibodies as described [35].
Immunoprecipitation experiments were carried out according to the instructions of the ProteinG Immunoprecipitation kit (Sigma). For each sample, 300 μg of total protein was immunoprecipitated with 2 μg of either anti-CDK2 (sc-163) or anti-CDK4 (c-22) antibody and 50 μl of protein G Sepharose beads (Sigma). The immunoprecipitated beads were washed and treated with ×2 SDS sample buffer, and heated at 95°C for 10 min. These samples were then separated on a 15% SDS gel by polyacrylamide gel electrophoresis and probed for the protein of interest. In vitro CDK4 kinase assays were performed as described [33]. All immunoblotting images were developed using an Amersham ECL plus western blotting detection system (GE Healthcare, Piscataway, NJ, USA) and processed in Alpha Innotech (San Leandro, CA, USA) gel imager. Bands were quantified using Spot Denso, Alpha Ease Fluor Chem SP (Alpha Innotech).
Quantitative real-time PCR
Total RNA was isolated using RNeasy (Qiagen, Valencia, CA, USA). cDNA synthesis was performed using random hexamers and it was reverse transcribed using Superscript II (Invitrogen) according to the manufacturer’s protocol. Real-time PCR was performed on an ABI 7000 sequence detection system using Taq-man gene expression assays (Applied Biosystems, Foster City, CA, USA). Primers were purchased from Applied Biosystems.
Islet morphometry, p21Cip subcellular localisation, proliferation and TUNEL analysis
Paraformaldehyde-fixed pancreatic tissues were embedded in paraffin using standard techniques. Sections were deparaffinised, rehydrated and incubated with blocking solution as previously described [12]. Sections were incubated overnight at 4°C with antibodies against insulin (Linco Research, St Charles, MO, USA), p21Cip (Santa Cruz Biotechnology and BD Biosciences), followed by secondary antibodies conjugated to FITC (Jackson Immunoresearch, West Grove, PA, USA). DAPI-containing mounting media (Vector Laboratories, Burlingame, CA, USA) was added to coverslips. Beta cell mass assessment was performed by point counting morphometry from five insulin-stained sections (5 μm) separated by 200 μm using NIH Image J software (v1.43f freely available at http://rsb.info.nih.gov/ij/index.html) as described [12]. Cell proliferation and apoptosis were analysed using Ki67 and TUNEL (Millipore) staining. TUNEL-positive and proliferating cells were identified by co-staining for TUNEL or antigen identified by monoclonal antibody Ki 67 (Ki67) and insulin. At least 1000–3000 stained cells were counted from each animal. For cell imaging of p21Cip in vitro, MIN6 and MIN6caAkt cells were allowed to attach onto coverslips and left to grow overnight before fixing with 4% paraformaldehyde at room temperature for 10 min, then permeabilising for 10 min with 0.1% Triton-X as previously described [36]. Three independent cultures were analysed. A cell was considered to have positive nuclear p21Cip staining when ∼80–90% staining was localised in the nucleus. The number of positive cells was calculated using Image J.
Metabolic studies—glucose, insulin and intraperitoneal glucose tolerance test
Fasting glucose levels were measured after an overnight fasting using an AccuChek II glucometer (Roche Diagnostics, Indianapolis, IN, USA). Fed glucose levels were obtained at the same time in the morning of each day. Glucose and insulin tolerance tests were performed by intraperitoneal delivery of 2 g/kg glucose or 0.75 U/kg insulin (Humalog, Eli Lilly, Indianapolis, IN, USA) to mice after 12 h fasting. Blood glucose was monitored for 2 h after glucose or insulin delivery. Plasma insulin levels were measured using rat insulin ELISA kit (Crystal Chem, Chicago, IL, USA).
Statistical analysis
All data are represented as the mean ± SEM from at least three independent experiments unless otherwise indicated. Data were analysed by Student’s t test or ANOVA followed by post hoc analysis, where appropriate. In some experiments, Student’s paired t test was used. Results were considered statistically significant when p < 0.05.
Results
Akt signalling regulates p21Cip transcription and protein stability
Previous experiments in transgenic mice overexpressing a constitutively active Akt mutant in beta cells (caAkt Tg) demonstrated that islets from these mice exhibit increased p21 Cip mRNA and protein levels [15]. To begin to assess the relationship of Akt and p21Cip, we first established an in vitro system using MIN6 cells producing green fluorescent protein (GFP) (control) or overexpressing a constitutively active form of Akt1 (MIN6caAkt). These cells exhibit increased Akt1, 2 and 3 (compared with augmented Akt1 and Akt2 in caAkt Tg islets) and elevated total activity, demonstrated by phosphorylation of GSK3β (electronic supplementary material [ESM] Fig. 1a,d). Similar to caAkt Tg mice, this cell line exhibits augmented proliferation resulting from increased levels of cyclin D2, D3 and CDK4 activity [33] (ESM Fig. 1 and data not shown). In addition, MIN6caAkt cells express higher levels of p21 Cip mRNA compared with controls (Fig. 1a). The changes in p21 Cip mRNA levels are similar to those observed in islets from caAkt Tg mice [15]. To further determine the contribution of stability on steady-state mRNA levels, we treated MIN6 and MIN6caAkt cells with the transcription inhibitor actinomycin D. The half-life of p21 Cip mRNA expression was significantly decreased in MIN6caAkt cells, suggesting that Akt induces p21 Cip mRNA levels mainly by transcriptional regulation (Fig. 1a). We next investigated p21Cip protein in MIN6caAkt cells and observed that these cells exhibited increased levels of p21Cip compared with control cells (Fig. 1c). The extent to which protein stability contributes to elevated p21 Cip expression was then determined by culturing MIN6 cells and islets with CHX. We observed that p21Cip protein levels decreased at a faster rate in control cells compared with MIN6caAkt (Fig. 1d). Figure 1e shows the quantification of p21Cip levels adjusted to the levels of this cell cycle inhibitor before CHX in MIN6caAkt and controls. To confirm that Akt regulates p21Cip protein stability in an ex vivo system, we performed similar experiments in islets from caAkt Tg mice. In agreement with our findings in MIN6 cells, p21Cip protein appears more stable in the presence of Akt signalling activation (Fig. 1f).
Akt regulates p21Cip levels by transcriptional regulation and protein stability. a Assessment of p21 Cip mRNA levels in MIN6GFP and MIN6caAkt cells using TaqMan RT-PCR. b p21 Cip mRNA stability assays from MIN6 and MIN6caAkt cells treated with 5 μg/ml actinomycin D in 5.5 mmol/l glucose. Half-lives were calculated using GraphPad Prism Software: MIN6GFP t ½ = 6.2 h; MIN6caAkt t ½ = 1.1 h; c Immunoblotting for p21Cip in MIN6GFP and MIN6caAkt cells. d p21Cip protein stability assessed by immunoblotting for p21Cip and actin in MIN6GFP and MIN6caAkt cells cultured with 12.5 μg/ml CHX for 0, 0.5, 1, 2, 4, 6 and 8 h. e Quantification of p21Cip stability experiments in MIN6GFP and MIN6caAkt cells. Protein bands for p21Cip were quantified and normalised to the levels in cells at time 0. f p21Cip protein stability assessed by immunoblotting for p21Cip and tubulin in islets cultured with 12.5 μg/ml CHX for 1 h. Data are presented as mean ± SEM of at least three independent experiments (n ≥ 3). *p < 0.05. In graphs: black line, MIN6GFP; grey line, MIN6caAkt. WT, wild type
Altered subcellular localisation of p21Cip in cells overexpressing Akt
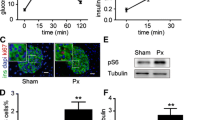
The subcellular localisation of p21Cip has been implicated to play a role in its stability and function [37]. Therefore, we tested whether the subcellular localisation of p21Cip is altered in cells overexpressing Akt. We observed that staining for p21Cip in control MIN6 cells was predominantly nuclear compared with MIN6caAkt cells (Fig. 2a, b). On the other hand, MIN6caAkt cells exhibited both nuclear and cytoplasmic staining but cytoplasmic p21Cip staining appeared to be more robust (Fig. 2a, b). Assessment of p21Cip staining in pancreases from caAkt Tg and control mice demonstrated that insulin-positive cells overexpressing Akt exhibit a predominantly cytoplasmic localisation of p21Cip compared with a more diffuse staining observed in wild-type islet cells (Fig. 2c). These data suggest that p21Cip subcellular localisation is altered in cells with elevated Akt signalling.
Altered subcellular localisation of p21Cip in cells overexpressing Akt. a Representative immunofluorescence imaging for p21Cip (red) and nuclear staining with DAPI (blue) in MIN6 and MIN6caAkt cells. b Quantification of p21Cip subcellular localisation. c Immunofluorescence imaging of endogenous p21Cip in pancreatic islets from non-transgenic and caAkt mice. Staining of p21Cip is shown in red and insulin in green. Data are presented as mean ± SEM of at least three independent experiments (n ≥ 3). *p < 0.05. NT, non-transgenic
MIN6 cells and islets overexpressing the caAkt mutant exhibit high levels of p21Cip bound to CDK4 and CDK2
We next set out to determine whether the increase in p21Cip levels via Akt modulation leads to greater assembly of the cyclin D/CDK4 complex or whether p21Cip preferentially binds to the cyclin E or A/CDK2 complex. To study this possibility, we first assessed the proportion of p21Cip bound to CDK4 and CDK2 in MIN6 cells and islets with activation of Akt signalling. Immunoprecipitation of CDK4 followed by immunoblotting for p21Cip demonstrated a twofold increase in p21Cip bound to CDK4 in MIN6caAkt (Fig. 3a). Similar experiments performed in isolated islets from wild-type and caAkt Tg mice also demonstrated twofold increase in p21Cip bound to CDK4 (Fig. 3b). The increase in the CDK4/p21Cip complex appeared to follow the same cellular distribution as p21Cip, with a more robust cytoplasmic fraction and a less abundant component in the nucleus (ESM Fig. 2a). Interestingly, p21Cip bound to CDK2 was also increased in MIN6caAkt cells and in islets from caAkt Tg mice (Fig. 3c,d). These studies suggest that p21Cip is an integral component of the cyclin D/CDK4 and cyclin E or A/CDK2 complexes in beta cells and that Akt signalling regulates the formation of these complexes. The functional effect of increased p21Cip localised to these complexes in beta cell proliferation and mass was then assessed in vivo.
Increased p21Cip levels bound to CDK4 and CDK2 complex in MIN6 cells and islets with activation of Akt signalling. a,b Assessment of p21Cip bound to the CDK4 complex in MIN6 (a) and in islets (b). c,d Assessment of p21Cip bound to the CDK2 complex in MIN6 (c) and in islets (d). Cell lysates from MIN6GFP and MIN6caAkt or islet lysates from non-transgenic and caAkt Tg were immunoprecipitated with CDK4 or CDK2 antibodies followed by immunoblotting for p21Cip. Quantification of p21Cip levels bound to CDK4 (a,b) and CDK2 (c,d). Immunoblotting for CDK4 and CDK2 were used as controls. Data are presented as mean ± SEM; n ≥ 3; *p < 0.05. In graphs, black bars, non-transgenic; grey bars, caAkt Tg. IP, immunoprecipitated; NT, non-transgenic
Metabolic assessment of p21Cip+/−/caAktTg mice
To assess whether the in vivo increase in p21 Cip mRNA expression and protein level plays a role as a cell cycle inhibitor or promotes proliferation by favouring the assembly of the cyclin D/CDK4 complex, we crossed caAkt Tg mice with mice deficient in p21 Cip. The effects of decreased p21Cip levels on glucose metabolism in caAkt Tg mice were then analysed in blood samples from 3–4-month-old mice in the fasted and fed states. Body weights among the different groups were comparable (Fig. 4a). No differences were observed in fasting or fed blood glucose levels between p21 Cip+/+, p21 Cip+/− and p21 Cip−/− mice (Fig. 4b,d). In contrast to the normal fasting and fed glucose levels in caAkt Tg /p21 Cip+/+ mice, caAkt Tg /p21 Cip+/− mice exhibited decreased blood glucose levels (Fig. 4b,d). Assessment of insulin values demonstrated that p21 Cip+/+, p21 Cip+/− and p21 Cip−/− mice exhibited comparable insulin levels in the fasting and fed states (Fig. 4c,e), yet serum fasting and fed insulin values of caAkt Tg /p21 Cip+/+ mice were elevated when compared with the non-transgenic groups (Fig. 4c, e). caAkt Tg /p21 Cip+/− mice exhibited a significant increase in fasting insulin levels compared to caAkt Tg /p21 Cip+/+ mice (Fig. 4c). These results indicate that fasting serum insulin levels are increased in caAkt Tg mice lacking one allele or deficient in p21 Cip. We were unable to obtain sufficient number of caAkt Tg /p21Cip−/− for these experiments.
Metabolic assessment of p21 Cip−/− and caAkt Tg intercross. a Body weight of 3-month-old male mice. Fasting glucose (b) and insulin (c) measurements were performed on overnight-fasted 3–4 month-old male non-transgenic mice or caAkt Tg containing two alleles (+/+) or one allele (+/−) in p21Cip mice. d,e Random glucose and insulin levels obtained in the same group and age of mice. f Intraperitoneal glucose tolerance tests were performed on the same group of mice. Data are presented as mean ± SEM (n = 8–10). *p < 0.05 compared with p21 Cip+/+; † p < 0.05 compared with caAkt Tg/p21 Cip+/+. p21 Cip+/+, grey circle; p21 Cip+/−, grey square; p21 Cip−/−, grey triangle; caAkt Tg, black triangle; and caAkt Tg/p21 Cip+/−, black square. NT, non-transgenic
To determine the alterations in glucose handling in these mice, we performed intraperitoneal glucose tolerance tests in 3–4-month-old mice. The glucose tolerance values in p21 Cip+/+, p21 Cip+/− and p21 Cip−/− mice were comparable (Fig. 4f). As described previously, caAkt Tg animals exhibited improved glucose tolerance at 30 and 60 min after glucose injection (Fig. 4f). As shown in Fig. 4b, fasting glucose levels were lower in caAkt Tg /p21 Cip+/− mice. Glucose tolerance in caAkt Tg mice lacking one allele of p21 Cip was improved when compared that of non-transgenic group. Interestingly, glucose levels at 30 and 60 min after glucose injection were lower in caAkt Tg /p21 Cip+/− mice when compared with caAkt Tg mice (Fig. 4f). These data suggest that a reduction in p21 Cip levels significantly improves glucose tolerance in caAkt Tg mice.
Deletion of one allele of p21Cip enhances beta cell mass in caAktTg mice
The results of the metabolic studies showed that a decrease in p21Cip levels alters the caAkt Tg phenotype and suggest that beta cell mass could be responsible for these observations. The histological appearance of the pancreas and quantification of beta cell mass were then assessed by islet morphometry. In adult pancreases, p21Cip+/− and p21Cip−/− mice exhibited beta cell mass comparable with that of p21Cip+/+ mice (Fig. 5a and quantitative analysis in Fig. 5b). Compared with p21Cip+/+, caAkt Tg mice demonstrated a sixfold increase in beta cell mass (Fig. 5a,b). Interestingly, the increase in beta cell mass observed in caAkt Tg was further enhanced in caAkt Tg/p21Cip+/− mice (Fig. 5a,b), which suggests that a reduction of p21Cip in caAkt Tg mice promoted beta cell proliferation. Collectively, these data point to the role of p21Cip as a negative regulator of the cell cycle that acts as a compensatory brake for beta cell expansion induced by Akt.
Determination of pancreatic and islet morphology of caAkt Tg and p21Cip intercross. a Representative pancreatic morphology from mice with different genotypes stained for insulin (red) and counterstained with haematoxylin (blue). b Beta cell mass in 4–5-month-old progeny from p21 −/− /caAkt Tg intercross (n ≥ 3). Data are mean ± SEM. *p < 0.05. NT, non-transgenic
Deletion of one allele of p21Cip in caAktTg mice enhances proliferation and survival
Because we observed an enhanced beta cell mass in caAkt Tg/p21 Cip+/− mice, we then analysed beta cell proliferation by Ki67 staining. We did not observe any difference in beta cell proliferation between p21 Cip+/−, p21 Cip−/− and p21 Cip+/+ mice (Fig. 6a). caAkt Tg mice exhibited a sixfold increase in beta cell proliferation when compared with p21Cip+/+ mice (Fig. 6a). Interestingly, caAkt Tg/p21 Cip+/− mice showed enhanced proliferation compared with caAkt Tg, which suggests that deletion of one allele of p21 Cip augments the proliferative response in caAkt Tg mice. Given the role of p21Cip in cell survival [38], we also assessed cell death. TUNEL staining analysis showed that caAkt Tg mice have increased cell death compared with p21 Cip+/+ control mice (Fig. 6b). However, caAkt Tg/p21Cip+/− mice exhibited less apoptosis compared with caAkt Tg mice, indicating that p21Cip enhances survival in conditions where there is activation of Akt signalling. Altogether, our data suggest that p21Cip levels modulate the proliferative and survival responses induced by overexpression of Akt in pancreatic beta cells.
Determination of beta cell proliferation and apoptosis among caAkt Tg and p21Cip intercrosses. a Proliferation index determined by percentage of Ki67-positive beta cells in p21 Cip and caAkt Tg intercross (n ≥ 3). TUNEL index determined by percentage of TUNEL-positive beta cells in p21 Cip and caAkt Tg intercross (n ≥ 3). Data are mean ± SEM. *p < 0.05. NT, non-transgenic
Discussion
The present study evaluated the mechanisms involved in the regulatory effect of p21Cip on proliferation induced by activation of Akt signalling in beta cells. We have shown that Akt activation increases p21Cip levels at least in part by increased transcription and regulation of protein stability. We have also shown that the subcellular localisation of p21Cip is altered in both MIN6caAkt cells and beta cells of caAkt Tg mice. The in vivo experiments using Akt transgenic mice suggest that even though the CDK4/cyclin D complex is formed, increased p21Cip levels exert a compensatory brake rather than increasing the assembly of the complex. These and published studies performed on p21 Cip-null mice [15] suggest that reduction of p21Cip levels is not sufficient to enhance beta cell proliferation. Instead, decreased p21Cip levels could favour beta cell cycle progression in conditions where there is increase in proliferation, such as in caAkt Tg mice. Therefore, our data also suggest that p21Cip could play an important role in the adaptation of beta cells in conditions where there is need for increased proliferation, as in conditions of insulin resistance.
The current studies show that p21Cip protein levels were elevated in cells and islets with enhanced Akt signalling, and this elevation results in part by enhanced transcription and increased protein stability. The actinomycin D experiments show that the half-life of p21 Cip mRNA was significantly reduced in MIN6caAkt cells supporting the concept that transcriptional regulation is the major mechanism by which p21 Cip RNA expression is increased by Akt. Previous studies have demonstrated that the tumour protein p53 and forkhead box O (FOXO) transcription factors are involved in p21 Cip transcription in different systems [39–43]. Akt could modulate transcriptional regulation of p21Cip by regulation of these transcription factors. Therefore, it is of future interest to test this possibility in vitro using promoter reporter assays. In addition to transcriptional regulation, treatment of both MIN6 cells and islets with CHX showed that the decay of p21 Cip was slowed in cells overexpressing a constitutively active Akt mutant. Thus, it appears that Akt also regulates p21Cip levels by increasing its protein stability. Our observation showing an increase in p21Cip stability is consistent with other studies showing that p21Cip is directly phosphorylated by Akt, resulting in increased protein stability and cytoplasmic localisation [44–46]. In accordance with this finding, we also observed increased cytoplasmic localisation of p21Cip in cells with enhanced Akt signalling. Conceivably, restriction of p21Cip to the cytosol could limit its effect on cell cycle inhibition. However, the increased fraction of CDK4 bound to p21Cip sequestered in cytoplasm could be, in part, a mechanism to indirectly limit unrestricted cell cycle progression in cells with activation of Akt. In addition to the effects on cell cycle progression, cytoplasmic p21Cip protects against apoptosis in tumoural cells [38, 47]. In contrast to this view, our results show that decreased p21Cip levels in beta cells with gain of Akt function augment survival. These results are intriguing and perhaps suggest that the function of cytoplasmic p21Cip in conditions of increased Akt activity could vary in vivo and be tissue specific. While the biological significance of elevated p21Cip levels and cytoplasmic localisation by Akt is still unclear, it is conceivable that this alteration may serve as a compensatory mechanism to prevent unrestricted proliferation.
An increasing body of evidence suggests that p21Cip may act as a positive regulator of the cell cycle that is transiently induced during G1–S progression as a result of mitogenic stimuli. Therefore, it was unclear whether p21Cip was repressing beta cell cycle progression by inhibition of cyclin E/CDK2 complex or promoting proliferation by activation of CDK4. To test this in vivo, we crossed caAkt Tg mice with p21 Cip-deficient mice. These experiments have shown that decreased p21Cip levels enhanced beta cell mass in caAkt Tg mice by increasing proliferation and enhancing survival. The changes in proliferation suggest that increased p21Cip levels in caAkt Tg mice could serve as a compensatory brake to prevent unrestricted proliferation. These results are in contrast to the normal beta cell mass and proliferation observed p21 Cip-deficient mice [48] and suggest that suppression of p21Cip levels is not sufficient to drive beta cell proliferation during basal conditions but could favour cell cycle progression during proliferative conditions, such as those observed in insulin-resistant states. It would be interesting to test the adaptation of p21 Cip-deficient mice in states of insulin resistance induced by high-fat feeding. This experiment was recently performed, but the beta cell proliferative response was not determined [48].
Metabolic characterisation of the caAkt Tg/p21 Cip intercross showed that deletion of p21 Cip resulted in additional alterations in carbohydrate metabolism. More specifically, reduction in p21Cip expression resulted in further increases in insulin levels and improvement in glucose tolerance on the caAkt Tg background. Interestingly, we observed that p21 Cip-deficient mice exhibited increased insulin sensitivity both on the non-transgenic or caAkt Tg background (data not shown). Therefore, it is likely that the increased insulin levels and improved glucose tolerance resulted predominantly from increases in beta cell mass. The changes in insulin sensitivity observed in these studies are intriguing and it is possible that alterations in adiposity in p21 Cip-null mice could play a role in peripheral insulin sensitivity [48, 49]. Conflicting results in adiposity in p21Cip-null mice have been reported, and it is unclear whether the effect of this cell cycle inhibitor on adiposity during basal conditions is similar to that observed in mice exposed to high-fat feeding.
The importance of p21Cip in regulation of the beta cell cycle is complex. Here, we have demonstrated that Akt increases p21Cip levels by modulating its protein stability and association with CDK4 and CDK2. In addition, we have demonstrated that the changes in p21Cip induced by Akt have a significant impact on regulation of beta cell mass, proliferation and survival. These data suggest that p21Cip levels could play an important role in inhibiting cell cycle progression in beta cells during proliferative responses. Thus, deregulation of p21Cip could limit the adaptation of beta cells to insulin resistance or states of beta cell regeneration.
Abbreviations
- Akt:
-
Protein kinase B
- CDK:
-
Cyclin-dependent kinase
- P21Cip :
-
CDK-interacting protein, CDK-inhibitor 1A
- CHX:
-
Cycloheximide
- GFP:
-
Green fluorescent protein
References
Dor Y, Brown J, Martinez OI, Melton DA (2004) Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46
Lu Y, Herrera PL, Guo Y et al (2004) Pancreatic-specific inactivation of IGF-I gene causes enlarged pancreatic islets and significant resistance to diabetes. Diabetes 53:3131–3141
Ueki K, Okada T, Hu J et al (2006) Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat Genet 38:583–588
Okada T, Liew CW, Hu J et al (2007) Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci USA 104:8977–8982
Hugl SR, White MF, Rhodes CJ (1998) Insulin-like growth factor I (IGF-I)-stimulated pancreatic beta-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. J Biol Chem 273:17771–17779
Withers DJ, Gutierrez JS, Towery H et al (1998) Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904
Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF (1999) Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet 23:32–40
Hennige AM, Burks DJ, Ozcan U et al (2003) Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J Clin Invest 112:1521–1532
Lingohr MK, Buettner R, Rhodes CJ (2002) Pancreatic beta-cell growth and survival—a role in obesity-linked type 2 diabetes? Trends Mol Med 8:375–384
Lingohr MK, Dickson LM, Wrede CE, McCuaig JF, Myers MG, Rhodes CJ (2003) IRS-3 inhibits IRS-2-mediated signaling in pancreatic beta-cells. Mol Cell Endocrinol 204:85–99
Tuttle RL, Gill NS, Pugh W et al (2001) Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med 7:1133–1137
Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA (2001) Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest 108:1631–1638
Garofalo RS, Orena SJ, Rafidi K et al (2003) Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 112:197–208
Cho H, Mu J, Kim JK et al (2001) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728–1731
Fatrai S, Elghazi L, Balcazar N et al (2006) Akt induces β-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 55:318–325
Rane SG, Dubus P, Mettus RV et al (1999) Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Genet 22:44–52
Tsutsui T, Hesabi B, Moons DS et al (1999) Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol Cell Biol 19:7011–7019
Malumbres M, Sotillo R, Santamaria D et al (2004) Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118:493–504
Kushner JA, Ciemerych MA, Sicinska E et al (2005) Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol Cell Biol 25:3752–3762
Chang F, McCubrey JA (2001) P21(Cip1) induced by Raf is associated with increased Cdk4 activity in hematopoietic cells. Oncogene 20:4354–4364
LaBaer J, Garrett MD, Stevenson LF et al (1997) New functional activities for the p21 family of CDK inhibitors. Genes Dev 11:847–862
Waga S, Hannon GJ, Beach D, Stillman B (1994) The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369:574–578
Weiss RH, Joo A, Randour C (2000) p21(Waf1/Cip1) is an assembly factor required for platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J Biol Chem 275:10285–10290
Kavurma MM, Khachigian LM (2004) Vascular smooth muscle cell-specific regulation of cyclin-dependent kinase inhibitor p21(WAF1/Cip1) transcription by Sp1 is mediated via distinct cis-acting positive and negative regulatory elements in the proximal p21(WAF1/Cip1) promoter. J Cell Biochem 93:904–916
Kavurma MM, Khachigian LM (2003) Sp1 inhibits proliferation and induces apoptosis in vascular smooth muscle cells by repressing p21WAF1/Cip1 transcription and cyclin D1-Cdk4-p21WAF1/Cip1 complex formation. J Biol Chem 278:32537–32543
Georgia S, Bhushan A (2006) p27 Regulates the transition of beta-cells from quiescence to proliferation. Diabetes 55:2950–2956
Rachdi L, Balcazar N, Elghazi L et al (2006) Differential effects of p27 in regulation of beta-cell mass during development, neonatal period, and adult life. Diabetes 55:3520–3528
Uchida T, Nakamura T, Hashimoto N et al (2005) Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 11:175–182
Cozar-Castellano I, Haught M, Stewart AF (2006) The cell cycle inhibitory protein p21cip is not essential for maintaining beta-cell cycle arrest or beta-cell function in vivo. Diabetes 55:3271–3278
Yang J, Zhang W, Jiang W et al (2009) P21cip-overexpression in the mouse beta cells leads to the improved recovery from streptozotocin-induced diabetes. PLoS One 4:e8344
Agudo J, Ayuso E, Jimenez V et al (2008) IGF-I mediates regeneration of endocrine pancreas by increasing beta cell replication through cell cycle protein modulation in mice. Diabetologia 51:1862–1872
Cozar-Castellano I, Weinstock M, Haught M, Velázquez-Garcia S, Sipula D, Stewart AF (2006) Evaluation of beta-cell replication in mice transgenic for hepatocyte growth factor and placental lactogen: comprehensive characterization of the G1/S regulatory proteins reveals unique involvement of p21cip. Diabetes 55:70–77
Balcazar N, Sathyamurthy A, Elghazi L et al (2009) mTORC1 activation regulates beta-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J Biol Chem 284(12):7832–7842
Bernal-Mizrachi E, Wice B, Inoue H, Permutt MA (2000) Activation of serum response factor in the depolarization induction of Egr-1 transcription in pancreatic islet beta-cells. J Biol Chem 275:25681–25689
Cozar-Castellano I, Harb G, Selk K et al (2008) Lessons from the first comprehensive molecular characterization of cell cycle control in rodent insulinoma cell lines. Diabetes 57:3056–3068
Alejandro EU, Johnson JD (2008) Inhibition of Raf-1 alters multiple downstream pathways to induce pancreatic beta-cell apoptosis. J Biol Chem 283:2407–2417
Child ES, Mann DJ (2006) The intricacies of p21 phosphorylation: protein/protein interactions, subcellular localization and stability. Cell Cycle 5:1313–1319
Cmielova J, Rezacova M (2011) p21(Cip1/Waf1) protein and its function based on a subcellular localization. J Cell Biochem 112:3502–3506
Herold S, Wanzel M, Beuger V et al (2002) Negative regulation of the mammalian UV response by Myc through association with Miz-1. Mol Cell 10:509–521
Seoane J, Le HV, Massague J (2002) Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature 419:729–734
Wu S, Cetinkaya C, Munoz-Alonso MJ et al (2003) Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene 22:351–360
Seoane J, Le HV, Shen L, Anderson SA, Massague J (2004) Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117:211–223
Evans-Anderson HJ, Alfieri CM, Yutzey KE (2008) Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res 102:686–694
Li Y, Dowbenko D, Lasky LA (2002) AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem 277:11352–11361
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC (2001) Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 3:245–252
Xia W, Chen JS, Zhou X et al (2004) Phosphorylation/cytoplasmic localization of p21Cip1/WAF1 is associated with HER2/neu overexpression and provides a novel combination predictor for poor prognosis in breast cancer patients. Clin Cancer Res 10:3815–3824
Coqueret O (2003) New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol 13:65–70
Inoue N, Yahagi N, Yamamoto T et al (2008) Cyclin-dependent kinase inhibitor, p21WAF1/CIP1, is involved in adipocyte differentiation and hypertrophy, linking to obesity, and insulin resistance. J Biol Chem 283:21220–21229
Naaz A, Holsberger DR, Iwamoto GA, Nelson A, Kiyokawa H, Cooke PS (2004) Loss of cyclin-dependent kinase inhibitors produces adipocyte hyperplasia and obesity. FASEB J 18:1925–1927
Acknowledgements
We acknowledge the MDRTC Cell and Molecular Biology Core (P60DK020572) and the Morphology Core at the University of Michigan Cancer Center for their services.
Funding
This work was supported by National Institutes of Health Grant DK-073716, DK084236 (to E. Bernal-Mizrachi), research grant from The Juvenile Diabetes Research Foundation and a Career Development Award from the American Diabetes Association (to E. Bernal-Mizrachi). E. U. Alejandro was supported by an NIH training grant (2T32DK071212-06).
Contribution statement
EUA and AS performed experiments, analysed data and wrote/edited the manuscript. MB-R, JOS, BG, AYC, LR, DJB, AW, APG and LE performed experiments, analysed data and edited the manuscript. EB-M conceived the study and edited the manuscript. All the authors approved the final version to be published.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Blandino-Rosano and E. U. Alejandro contributed equally to this study.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
ESM Fig. 1
Characterisation of MIN6 cells overexpressing caAkt mutant (MIN6 caAkt ) and controls (MIN6 GFP ). a. Immunoblotting for HA and phospho GSK3β (Ser9). b. Immunoblotting for cell cycle components. c. In vitro kinase assays for CDK4 activity using Retinoblastoma protein as substrate in MIN6caAkt, MIN6GFP and MIN6 cells after stimulation of IGF-I (100nm). d. Immunoblotting for Akt isoforms in MIN6 cells and islets from caAkt Tg. Data are mean ± SE. *p < 0.05 (n ≥ 3). (PDF 7,455 kb)
ESM Fig. 2
Sub-cellular localisation of CDK4 in cells overexpressing Akt. a. Immunostaining for p21Cip (red), CDK4 (green) and nuclear staining with DAPI (blue) in MIN6 and MIN6caAkt cells. (PDF 23377 kb)
Rights and permissions
About this article
Cite this article
Blandino-Rosano, M., Alejandro, E.U., Sathyamurthy, A. et al. Enhanced beta cell proliferation in mice overexpressing a constitutively active form of Akt and one allele of p21 Cip . Diabetologia 55, 1380–1389 (2012). https://doi.org/10.1007/s00125-012-2465-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2465-9