Abstract
Aim/hypothesis
Glucocorticoid hormones (GCs) are widely used to treat a variety of inflammatory and immune diseases. However, their long-term administration is associated with adverse metabolic effects, including glucose intolerance and diabetes. Our objective was to elucidate the mechanisms by which GCs affect beta cell survival with a specific emphasis on the role of the thioredoxin-interacting protein (TXNIP) in beta cell apoptosis.
Methods
Human and mouse islets, together with MIN6 beta cells, were exposed to dexamethasone (Dex) and apoptosis was assessed by measuring the percentage of sub-G1 cells, the appearance of cleaved caspase-3 or by using a TUNEL assay. Dex-upregulated expression of Txnip mRNA was analysed by real-time PCR, and GC-modulated production and modification of proteins were determined by western blotting.
Results
We provide evidence that TXNIP, a negative regulator of the antioxidant thioredoxin (TRX), is strongly induced in beta cells by GCs and that its induction is dependent on p38 mitogen-activated protein kinase (MAPK) activation. TXNIP downregulation by RNA interference, overexpression of the radical scavenger TRX1 or elevation of intracellular cAMP levels attenuated the Dex-mediated apoptosis. Dex-induced Txnip expression and beta cell apoptosis are mediated by the glucocorticoid receptor (GR), as the GR antagonist RU486 fully abolishes these effects.
Conclusions/interpretation
Altogether, our data suggest TXNIP as a novel mediator of GC-induced apoptosis in beta cells and further contribute to our understanding of beta cell death pathways.
Similar content being viewed by others
Introduction
Glucocorticoids (GCs) are widely used to treat a variety of inflammatory and immune diseases [1]. However, they are also associated with adverse metabolic effects, including glucose intolerance and diabetes [2, 3]. Cumulative evidence indicates that a balance exists between insulin resistance and an effective beta cell mass. Beta cells adequately adapt to changes in insulin sensitivity by varying insulin secretion, thus maintaining euglycaemia. However, in susceptible individuals, when the beta cell mass fails to compensate for insulin resistance, type 2 diabetes ensues. Indeed, beta cell dysfunction has been acknowledged as a key defect underlying the development of type 2 diabetes [4]. In GC-induced impaired glucose tolerance, the compensatory increase in plasma insulin appears to be attenuated by the direct inhibitory effect of GCs on insulin secretion, as suggested both by in vivo and in vitro studies [5–8]. Several reports indicate that the effects of GCs on beta cell function are highly dependent on duration of exposure, dosage and susceptibility of the population exposed [9]. Healthy volunteers treated with high-doses of oral prednisolone showed acutely impaired insulin secretion [10, 11]. A few studies have also reported the role of these GCs during pancreatic development [12]. In normally nourished rat fetuses, increased beta cell mass was associated with low corticosterone levels, while decreased beta cell mass was observed under conditions of fetal overexposure to GCs [13]. In fact, the conditional inactivation of the glucocorticoid receptor (GR) in all pancreatic progenitors (GR-Pdx1-Cre mice) led to a doubling of beta cell mass [14]. GCs have also been shown to suppress insulin [15] and Pdx1 [16] gene expression in beta cells. Taken together, this research shows that GCs affect both embryonic beta cell development and beta cell function in the adult through interference with various signalling pathways, leading to impaired beta cell function and ultimately to apoptosis [6–9, 17, 18].
The aim of this study was to better understand the mechanisms by which GCs induce apoptosis in beta cells. Still little is known about the identity of the target genes whose transactivation or transrepression are involved in GC-induced beta cell apoptosis. In an attempt to further elucidate this process, we tested the role of the TXNIP gene [19]. TXNIP, also known as vitamin D3-upregulated protein 1 (VDUP-1) and thioredoxin-binding protein-2 (TBP-2), is a negative regulator of the antioxidant thioredoxin (TRX) [20]. Cumulative evidence suggests a link between TRX and mammalian metabolism through the actions of TXNIP. Txnip-null mice are hypoglycaemic, hypoinsulinaemic, and show blunted glucose production following a glucagon challenge, consistent with a central liver glucose-handling defect [21]. Studies in pancreatic beta cells have shown that TXNIP functions as an effector of glucotoxicity, where high glucose stimulates [22], and insulin represses its expression [23]. The glucose-induced increase in Txnip transcription is mediated by a carbohydrate response element binding protein (ChREBP) [24]. Beta cell-specific Txnip deletion (bTKO) was found to enhance beta cell mass and reduce apoptosis in streptozotocin-treated mice. Moreover, TXNIP deficiency leads to increased protein kinase B (Akt)/apoptosis regulator BCL-X (Bcl-xL) signalling and reduced mitochondrial beta cell death, suggesting that these mechanisms may mediate the beta cell protective effects in TXNIP-deficient cells [25, 26].
GCs are known to induce apoptosis and reduce proliferation in a variety of cell types by targeting distinct intracellular signalling pathways depending on the cell system and on the responsiveness of the apoptotic machinery [27, 28]. The gene expression profile of dexamethasone (Dex)-treated WEHI7.2 T cell lymphoma cells revealed that TXNIP mRNA was significantly upregulated. It was further demonstrated that TXNIP is involved in mediating glucocorticoid-induced apoptosis in these cells and in adult T cell leukaemia [29].
In an attempt to identify genes directly affected by GCs and involved in eliciting the apoptotic process in beta cells, we present evidence that GCs strongly induce the expression of the redox regulatory protein TXNIP/VDUP-1 in both human and mouse islets, as well as in MIN6 beta cells. While overexpression of Txnip was sufficient to induce apoptosis, its repression significantly inhibited Dex-induced apoptosis. Both Dex-induced Txnip expression and apoptosis in beta cells are mediated through the GR and depend on the activation of the p38 MAPK pathway. Together, our data suggest TXNIP as one of the mediators of GC-induced apoptosis in beta cells.
Methods
Reagents
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; thiazolyl blue (MTT), mifepristone (RU486), forskolin, IBMX (isobutylmethylxanthine) and 1α,25-dihydroxyvitamin D3 (VitD3) were purchased from Sigma-Aldrich (St Louis, MO, USA); methylprednisolone sodium succinate was purchased from Pfizer, Belgium. Antibodies against TXNIP/VDUP-1 were from Zymed (Invitrogen, Grand Island, NY, USA), antibodies to TRX1, cleaved caspase-3 (Asp175), phospho-p38 MAPK (Thr180/Tyr182), and Akt and phospho-Akt (Ser473) were from Cell Signaling Technology (Danvers, MA, USA) and α-tubulin from Sigma-Aldrich. Sodium phosphate dexamethasone (Dex) solution was from the Mylan pharmaceutical company, and the p38 inhibitor SB203580 was purchased from AG Scientific (San Diego, CA, USA).
Cell culture and islet tissue isolation
Mouse MIN6 beta cells, kindly provided by J.-I. Miyazaki (University of Osaka, Japan), and rat INS1 cells were cultured in DMEM 2g/l and RPMI 1640 medium (Biological Industries, Kibbutz Beit Haemek, Israel), with 13% and 10% heat inactivated fetal bovine serum (FBS) respectively and 60 μmol/l beta-mercaptoethanol. Mouse α-TC6.1 glucagonoma cells were maintained in DMEM 4.5g/l and 10% FBS. Culture media were supplemented with 2 mmol/l l-glutamine, penicillin (100 U/ml) and streptomycin (100 μg/ml). Mouse islets were isolated from C57Bl/6 mice and cultured as described previously [30]. Human islets were obtained from the European Consortium for Islet Transplantation (ECIT) at the San Raffaele Hospital (Milan, Italy).
MTT cell viability assay and TUNEL assay
The viability of cells was evaluated using 7 × 104 MIN6 cells cultured in 24-well plates. On the following day, the cells were incubated with the indicated treatments. During the last 30 min of incubation, MTT solution was added at a final concentration of 0.15 g/l. After removing the medium, 500 μl of dimethylsulfoxide was added to each well to dissolve the formazan. The absorbance was read in an ELISA reader at 550 nm. Viability was calculated in comparison with control cells. Each treatment was performed in triplicate. The TUNEL assay was performed as previously described [30].
Cell cycle analysis
MIN6 cells were seeded in six-well plates and, the following day, the medium was changed to fresh medium in the presence or absence of Dex. At the end of incubation, the floating cells, as well as the trypsinised adherent cells, were collected. Following centrifugation, the cells were fixed in ice-cold methanol, rehydrated in PBS, treated with 50 μg/ml DNase-free RNase, and incubated in 50 μg/ml propidium iodide for 30 min prior to cell cycle analysis by flow cytometry (FACStar, Becton Dickinson, Franklin Lakes, NJ, USA). Ten thousand events were counted for each sample, where sub-G1 cells are considered apoptotic cells. In transfection studies with GFP fusion proteins, a separate cell cycle analysis was done on the GFP positive (‘transfectant’) and the GFP negative (‘non-transfectant’) cell populations of the same sample. Analysis was performed using Cellquest Software.
Western blotting
Cells were lysed in Laemmli protein sample buffer, separated on SDS-polyacrylamide gel, and transferred to 0.2 μm nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). After blocking in 5% dried skimmed milk, the membranes were first incubated with the appropriate antibodies, and then with horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG, followed by analysis with an enhanced chemiluminescence detection kit (Biological Industries).
siRNA electroporation
MIN6 cells were suspended in 100 μl of Amaxa nucleofector solution and electroporated with 1 μmol/l Txnip siRNA or control siRNA (Invitrogen) using Amaxa Nucleoporator (Lonza, Walkersville, MA, USA). The cells were transferred into fresh medium immediately after electroporation and cultured for 24 h prior to treatments.
Cell transfection
MIN6 cells were seeded in 12-well plates and, on the following day, cells were transfected in Opti-MEM medium (GIBCO, Invitrogen) with either human TRX1-Gfp or mouse Txnip-Gfp using Lipofectamine 2000 (Invitrogen). Twenty hours later, fresh medium was added in the presence or absence of Dex.
Real-time PCR
RNA was isolated from cells by using TRIzol reagent (Invitrogen) and reverse transcribed into cDNA using the Masterscript kit (5 PRIME, Hamburg, Germany) and random primers (Applied Biosystems, Carlsbad, CA, USA). Real-time PCR was performed using Platinum SYBR Green qPCR SuperMix-UDG with ROX reference dye (Invitrogen). The mouse primers used are presented as electronic supplementary material (ESM) Table 1.
Statistical analysis
The data are presented as mean ± SD. Student’s t test was used to compare treated samples with untreated control samples. A p value <0.05 was considered statistically significant.
Results
GCs induce apoptosis of beta cells
MIN6 beta cells were exposed to various concentrations of Dex (1-500 nmol/l) for 24–72 h, and the cell viability was determined by MTT assay. Figure 1a shows that Dex significantly decreased beta cell survival with time and a maximal effect was observed with 100 nmol/l Dex. This effect was also observed when MIN6 cells were exposed to methylprednisolone (Fig. 1b). To validate whether the deleterious effect of Dex on cell survival was specific to beta cells, MIN6 and INS1 insulinomas, as well as the αTC6.1 glucagonoma cell line, were exposed to 100 nmol/l Dex for 48 h and cell viability was measured. The results presented in Fig. 1c–e confirm that Dex significantly decreased the viability of MIN6 (30 ± 6 %) and INS1 (25 ± 4 %) beta cells, but had barely any effect on the viability of αTC6.1 or that of the human PANC1 pancreatic ductal carcinoma cells (data not shown).
Dose- and time-dependent effect of GCs on cell viability. a MIN6 cells were exposed for 24 h (white bars), 48 h (black bars) or 72 h (hatched bars) to increasing concentrations of Dex. b MIN6 cells were exposed for 48 h (black bars) and 72 h (hatched bars) to 167 nmol/l methylprednisolone (Pred). c MIN6, d INS1 insulinoma cells and e α-TC6.1 glucagonoma cells were treated for 48 h with 100 nmol/l Dex (+). Cell viability was evaluated by using the MTT assay. Values are the average of at least three experiments ± SD, ***p < 0.001 vs unexposed control
To elucidate whether the decrease in cell viability is, at least in part, due to beta cell apoptosis, we analysed the DNA content of Dex-treated MIN6 cells for 48 or 72 h by flow cytometry and measured the sub-G1 DNA peak associated with apoptotic cells carrying hypodiploid DNA content. The results presented in Fig. 2a demonstrate that Dex treatment leads to an increase in the percentage of cells undergoing apoptosis. Further evidence for Dex-mediated apoptosis comes from western blot analysis demonstrating the activation of caspase-3 in MIN6 cells exposed to the hormone for 16, 48 or 72 h (Fig. 2b). The effects of dexamethasone on islet beta cell dysfunction depend on the duration and dose of exposure [3, 9, 31]. Since Dex-induced apoptosis of pancreatic islet beta cells is detected at significant levels following long-term exposure, we incubated isolated mouse islets with 100 nmol/l Dex for 9 days and the prevalence of DNA damage in beta cells was evaluated by labelling DNA breaks, which are a characteristic event occurring in the late stages of apoptosis. Histological analysis of apoptotic nuclei co-stained for insulin confirmed that beta cells are more susceptible to Dex-induced apoptosis (13.5% apoptotic beta cells/islet 4.8% in untreated islets; Fig. 2c).
Dex induces apoptosis in MIN6 and mouse islet beta cells. a MIN6 cells were exposed to 100 nmol/l Dex for 48 h (black bars) or 72 h (hatched bars) and analysed by flow cytometry. The percentage of the sub-G1 cell population represents apoptotic cells. The data represent an average ± SD of three independent samples. †p < 0.0007. b MIN6 cells were treated with 100 nmol/l Dex for the indicated time periods and processed for western blot analysis using antibodies specific to cleaved caspase-3 or α-tubulin. c Isolated mouse islets were treated with 100 nmol/l Dex (+) for 9 days and the percentage of apoptotic nuclei was evaluated using a TUNEL assay in cells co-stained for insulin. *p < 0.05
Dexamethasone affects TXNIP/VDUP-1 and TRX expression
In an attempt to clarify the mechanisms involved in Dex-induced beta cell death, we investigated the potential role of TXNIP in this apoptotic process. TXNIP, initially identified as VDUP-1 [19], was later shown to be upregulated in a mouse lymphoma cell line [32] by Dex. To study whether Dex could also upregulate TXNIP in MIN6 cells, these cells were treated with either increasing concentrations of Dex or VitD3 for 48 h or with 100 nmol/l Dex for various time periods. VitD3 was used here as a positive control for TXNIP production. The TXNIP/VDUP-1 protein levels were analysed by western blot and the results presented in Fig. 3a–c show an accumulation of TXNIP protein both with increasing concentrations of Dex (Fig. 3a) or VitD3 (Fig. 3b) and with time (Fig. 3c). The induction of Txnip gene expression with time was confirmed by real-time PCR analysis of mRNA levels in MIN6 cells treated with 100 nmol/l Dex for various time periods (Fig. 3d). TXNIP was also significantly induced in human and mouse islets by 100 nmol/l Dex (Fig. 3 e, f). Altogether, our data show a strong activation of Txnip gene expression by Dex, similar to that obtained with VitD3.
Dex induces TXNIP production in MIN6 beta cells, and mouse and human islets. a and b MIN6 cells were exposed to various concentrations of Dex or VitD3 as indicated for 48 h prior to western blot analysis using antibodies to TXNIP or α-tubulin. c Time-dependent production of TXNIP in MIN6 cells exposed to 100 nmol/l Dex (+) for the indicated time periods. d Real-time PCR analysis of Txnip mRNA expression in MIN6 cells exposed to 100 nmol/l Dex (black bars) for the indicated time periods. Gapdh mRNA was used as internal standard. The average of three separate experiments ± SD is presented as fold increase relative to untreated cells (control, white bars). *p < 0.05. e Human islets were exposed to 100 nmol/l Dex (+) for 4 days prior to western blot analysis using antibodies to TXNIP or α-tubulin. f Mouse islets were exposed to 11 mmol/l glucose in the absence (–) or presence (+) of 100 nmol/l Dex or to 25 mmol/l glucose for 3 days as indicated, prior to western blot analysis using antibodies to TXNIP or α-tubulin
As glucose is known to stimulate Txnip expression in beta cells [24], we compared the ability of Dex to induce TXNIP in mouse islets with that of high glucose (25 mmol/l). A robust induction was observed with both treatments (Fig. 3f).
Dex-induced Txnip expression and apoptosis are mediated through the GR
To test the direct involvement of the GR in beta cell death, MIN6 cells were exposed to 100 nmol/l of Dex in the presence or absence of 1 μmol/l RU486, a GR antagonist. RU486 fully prevented beta cell death as measured by MTT assay (Fig. 4a) and the level of cleaved caspase-3 (Fig. 4b). Furthermore, RU486 blocked the induction of TXNIP protein synthesis by Dex (Fig. 4b), indicating that Dex-stimulated TXNIP production depends on GR activation. RU486 also significantly inhibited the Dex-mediated reduction in TRX1 levels observed in MIN6 cells (Fig. 4b). Of note, the reduction of TRX1 protein by Dex occurred despite no significant alterations in Trx1 mRNA levels (data not shown).
The GR antagonist RU486 prevents TXNIP induction and TRX downregulation and protects MIN6 cells from Dex-induced apoptosis. a MIN6 cells were treated with 100 nmol/l Dex and/or with 1 μmol/l RU486 for 48 h and cell viability assessed by MTT assay. Values represent the average of at five experiments in quadruplicate ± SD, ***p < 0.001. b Western blot analysis of MIN6 cells treated with 100 nmol/l Dex and/or 1 μmol/l RU486 for 48 h using antibodies directed against the indicated proteins
Altogether, these data indicate that GCs have a potentially negative effect on the redox regulatory mechanisms of MIN6 cells. On the one hand, Dex induces the expression of TXNIP which inhibits TRX1 activity [14, 20, 21], and on the other hand, Dex decreases TRX1 protein levels, thereby further reducing the redox-regulating ability of the cell. The GR antagonist RU486 prevented these effects, thus rescuing beta cells from Dex-induced apoptosis.
TXNIP potentiates and TRX1 attenuates apoptosis in MIN6 cells
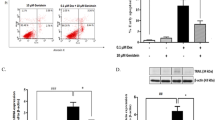
To further determine the contribution of TXNIP in Dex-induced beta cell apoptosis, an expression vector encoding the mouse Txnip gene fused to that of enhanced green fluorescent protein (Txnip-Gfp) was transiently expressed in MIN6 cells. Twenty hours post-transfection, the cells were incubated for an additional 48 h in the presence or absence of 100 nmol/l Dex. DNA content and TXNIP-GFP levels were simultaneously analysed by flow cytometry. The percentage of sub-G1 cells in the GFP positive subpopulation was compared with that of the GFP negative cells within the same cell culture. DNA fragmentation (sub-G1 DNA content) indicative of apoptotic cells was detected in 20.4 ± 3.42% of cells producing TXNIP-GFP, in comparison with only 8.2 ± 1.39% in the GFP negative subpopulation (Fig. 5a, p < 0.005). These values were further increased in the presence of Dex.
TXNIP potentiates, and TRX1 attenuates apoptosis in MIN6 beta cells. MIN6 cells were transfected with either Txnip-Gfp (a) or TRX1-Gfp (b). Twenty hours post-transfection, the cells were incubated for an additional 48 h in the presence or absence of Dex. Cells were then collected and for each sample, cell cycle analysis was performed separately on TXNIP-GFP-positive cells (black bars) and TXNIP-GFP-negative cells (white bars), and the percentage of sub-G1 cells determined by flow cytometry analysis. The data are the average of three to five independent transfections ± SD, †p < 0.005. c Txnip downregulation in MIN6 cells attenuates Dex-induced apoptosis. MIN6 cells were electroporated with Txnip siRNA or control siRNA (con). Cells were exposed the following day to 100 nmol/l Dex (+) for 48 h. Protein extracts were analysed by western blot using antibodies directed against the indicated proteins. d Cell viability was evaluated by using the MTT assay. Values are the average of four to eight experiments ± SD, ***p < 0.001
Since TXNIP associates with reduced TRX [20], it could exert its pro-apoptotic effect at least in part by inhibiting TRX activity. To test this hypothesis in Dex-induced beta cell death, MIN6 cells were transiently transfected with TRX1-GFP. Following transfection, the cells were incubated with or without 100 nmol/l Dex. Figure 5b shows that cells producing TRX1-GFP partially rescued MIN6 cells from Dex-induced apoptosis (11.09 ± 3.9% vs 24.3 ± 4.8%; p < 0.02). These findings indicate that TXNIP alone is sufficient to induce apoptosis and that overexpression of the radical scavenger TRX1 can, to a certain degree, protect beta cells from Dex-induced apoptosis.
Downregulation of endogenous Txnip expression reduces the sensitivity of MIN6 cells to Dex-mediated apoptosis
To further investigate the involvement of endogenous TXNIP in Dex-induced apoptosis of beta cells, RNA interference was used to repress Txnip expression. To this end, MIN6 cells were electroporated with Txnip siRNA or control siRNA prior to Dex treatment for 48 h. Knockdown of Txnip attenuated the Dex-induced apoptosis of MIN6 cells as demonstrated by reduced activation of caspase-3 on western blot (Fig. 5c). In addition, cell viability was determined by the MTT assay and Fig. 5d shows a mild increase in the survival of Dex-treated beta cells producing lower TXNIP levels (64% vs 77% p < 0.0007). These results support our hypothesis that TXNIP mainly contributes to Dex-induced beta cell death.
Elevated intracellular cAMP levels prevent the effects of Dex-induced Txnip expression on beta cell viability
Increased intracellular cAMP levels have previously been shown to attenuate Dex-induced beta cell death using exendin-4 and forskolin [18, 33]. This urged us to study the effect of the phosphodiesterase inhibitor IBMX or the adenylate cyclase activator forskolin in MIN6 cells. The elevated cAMP levels significantly reduced Dex-stimulated Txnip expression both at the protein (Fig. 6a) and at the RNA levels (Fig. 6 c, d).
Increased intracellular cAMP prevents Dex-induced TXNIP production and beta cell death and restores Akt phosphorylation. a, b MIN6 cells were exposed to 100 nmol/l Dex (+) for 6, 12 or 24 h in the presence (+) or absence (−) of 200 μmol/l IBMX prior to western blot analysis using antibodies to TXNIP or α-tubulin (a); to phospho-Akt (Ser 437) (p-Akt) or total Akt (b). c, d Real-time PCR analysis of Txnip mRNA expression in MIN6 cells exposed to 100 nmol/l Dex (hatched and dotted bars) for 24 h in the presence (+) or absence (−) of 10 μmol/l forskolin (c) or 200 μmol/l IBMX (d). Gapdh mRNA was used as internal standard. The average of three separate experiments ± SD is presented as fold increase relative to untreated cells. *p < 0.05. e, f MIN6 cells were exposed to 100 nmol/l Dex (hatched and dotted bars) for 48 h in the presence (+) or absence (−) of 10 μmol/l forskolin (e) or 200 μmol/l IBMX (f). Cell viability was evaluated by using the MTT assay. Values are the average of at least three experiments in duplicate ± SD, †p < 0.03
Under these conditions, increased cAMP also prevented the reduction in Akt activation (phospho-Akt, S473) caused by Dex treatment (Fig. 6b) and also protected MIN6 cells from Dex-reduced cell viability (Fig. 6e, f).
Induction of Txnip in GC-treated cells depends on the p38 MAPK pathway
Next, we investigated the signalling mechanisms involved in GC-induced TXNIP expression. MIN6 cells incubated with Dex showed an increase in p38 MAPK phosphorylation, which remained at relatively high levels up to 24 h after stimulation (Fig. 7a). To assess whether p38 MAPK activation regulates Txnip expression, MIN6 cells were pretreated with 20 μmol/l of the p38 inhibitor SB203580 for 60 min or with DMSO prior to Dex treatment for 72 h. The p38 inhibitor reduced the GC-induced TXNIP protein levels by 50% and blocked the activation of caspase-3 by 70% (Fig. 7b). Moreover, it partially blunted the Dex-reduced MIN6 cell viability, which increased from 71% to 80% (p < 0.04) and from 48% to 61% (p < 0.002) following 48 or 72 h Dex treatment, respectively (Fig. 7c) as determined by the MTT assay. The weaker effect of p38 inhibition on MTT assay indicates that Dex-mediated p38 MAPK is specifically involved in GC-induced Txnip expression and apoptosis, while slightly relieving the metabolic impairments associated with GC excess.
Dex-induced p38 activation contributes to TXNIP induction and beta cell death. a MIN6 cells were exposed to 100 nmol/l Dex (+) for 4, 8, 12 and 24 h prior to western blot analysis using antibodies to phospho-p38 (Thr 180/Tyr 182), or α-tubulin. b MIN6 cells were exposed to 100 nmol/l Dex (+) for 72 h in the presence (+) or absence (−) of the p38 MAPK inhibitor SB203580 and then analysed by western blot using the indicated antibodies. c MIN6 cells were exposed to 100 nmol/l Dex for 48 h (white bars) or 72 h (black bars) in the presence (+) or absence (−) of the inhibitor SB203580 and cell viability was evaluated by MTT assay. Values are the average of at least three experiments ± SD, †p < 0.002
Discussion
Glucocorticoids (GCs), which activate GR signalling and thus modulate gene expression, are widely used to treat immune-mediated diseases [1]. The role of GCs in beta cell function has been extensively studied in both humans and rodents [3]. In healthy men, it was demonstrated that acute and chronic exposure to prednisolone led to impaired beta cell function in addition to reducing insulin sensitivity [10, 11]. In transgenic mice specifically overexpressing the GR in beta cells, GCs inhibited insulin release in vivo, suggesting that the pancreatic beta cell is a direct target of the diabetogenic effect of GCs [6]. Interestingly, these animals develop diabetes with age [34]. In vitro, several studies have shown that rodent-derived islets decreased glucose-stimulated insulin release following both acute and prolonged exposure to various GC analogues [7, 9, 17, 31, 35–38]. In addition to perturbing the process of insulin secretion, GCs were shown to decrease beta cell specific gene expression and induce apoptosis [18, 33, 39]. However, it is still unclear which of the genes regulated by GCs contribute to beta cell death. One of the GR-regulated genes is TXNIP, which was originally found to be upregulated by glucose in human islets using transcription profiling (Shalev et al. [40]; L. Havin and L. Amior, unpublished data). The effect of glucose on gene expression was shown to be mediated by ChREBP [24], and increased levels of TXNIP were associated with glucotoxicity [22–24, 26, 41].
In the present study, we provide new evidence aimed at elucidating the mechanisms involved in GC-induced apoptosis of beta cells. We demonstrate that Dex strongly induces the expression of TXNIP/VDUP-1 in both MIN6 and INS1 beta cell lines as well as in normal human and mouse pancreatic islets. In this study we show that, in mouse islets and in MIN6 cells, Dex appears to be a slightly more potent inducer of TXNIP than high glucose (25 mM).
The increased GC-mediated Txnip expression with time was accompanied by a concomitant induction of beta cell death. Apoptosis was shown to be one of the mechanisms responsible for the observed reduced beta cell viability, as determined by cleaved caspase-3 and the increased percentage of sub-G1 cells. Indeed, overexpression of Txnip in MIN6 cells increased both basal and Dex-induced apoptosis. This is consistent with previous findings regarding the role of TXNIP in GC-induced apoptosis in T cells [29, 31] and in beta cells [22]. TXNIP is known to associate with reduced TRX, one of the major pro-survival thiol reducing systems in the cells [20] and could exert its pro-apoptotic effect at least in part by blocking TRX activity. Our study shows that MIN6 cells overexpressing the radical scavenger TRX1 partially rescued MIN6 cells from Dex-induced apoptosis. The role of TRX in protecting beta cells in both type 1 and type 2 diabetes has been reported. Indeed, beta cell specific expression of Trx1 in NOD mice slows the development of the disease, without preventing insulitis [42]. In the db/db mouse model of type 2 diabetes, overexpression of Trx1 significantly suppressed the progression of hyperglycaemia [43]. TRX1 prevented the reduction of the pancreatic and duodenal homeobox 1 (PDX1) and V-maf musculoaponeurotic fibrosarcoma oncogene homologue A (MafA) transcription factors as well as islet insulin content in these mice. In contrast to the reciprocal pattern of expression between Txnip and Trx1 mRNA previously reported [44–46], the Dex-reduced TRX1 protein levels in GC-treated MIN6 cells occurred despite no significant alterations in Trx1 mRNA (data not shown).
We further demonstrate the contribution of TXNIP in GC-induced beta cell apoptosis by decreasing its expression using siRNA. MIN6 cells transfected with Txnip siRNA showed a significant decrease in caspase-3 activation. In agreement with our results, TXNIP deficiency protected pancreatic beta cells from glucose toxicity and apoptosis [22] and rescued mice from streptozotocin-induced diabetes. Moreover, TXNIP deficiency prevented the development of type 2 diabetes in ob/ob mice through preservation of beta cell mass and function [25].
Cumulative evidence indicates that activating cAMP signalling attenuates GC-induced beta cell dysfunction and prevents apoptosis [11, 18, 47, 48]. Here, we present evidence that elevated intracellular cAMP levels also rescue MIN6 beta cells from the negative effects of Dex on cell viability, concomitant with a decrease in TXNIP protein levels. We further show that elevated intracellular cAMP levels prevent the Dex-induced reduction in phospho-Akt levels. Our findings accord with previous studies showing that the glucagon-like peptide 1 (GLP-1) analogue exendin-4, which increases cAMP concentrations, antagonises Dex-induced apoptosis of INS1 beta cells [18]. Glucocorticoids were shown to increase phosphodiesterase (PDE) activity leading to reduced intracellular cAMP levels [47]. More recently, this group reported that cAMP signalling stimulates proteasome degradation of glucose-induced TXNIP in INS1 beta cells [48]. It is therefore conceivable that elevated intracellular cAMP levels (by either stimulating PDE activity using the inhibitor IBMX, or adenylate cyclase using forskolin, exposing beta cells to GLP-1 or to its analogue exendin-4) reverse the Dex-inhibitory effect on Akt signalling and induce the degradation of TXNIP, reducing its pro-apoptotic effect, leading to increased beta cell survival.
Another important finding of our study is that the upregulation of TXNIP by Dex is dependent on p38 MAPK activation. Prevention of the sustained activation of the p38 pathway occurring after Dex treatment significantly reduced both Txnip expression and caspase-3 activation, and restored, to a certain extent, MIN6 cell viability. The weaker effect of p38 inhibition on the MTT assay indicates that Dex-mediated p38 MAPK is mainly involved in GC-induced Txnip expression and apoptosis, while slightly relieving the metabolic impairments associated with GC excess. p38 MAPK has also been documented to be involved in TXNIP upregulation by high glucose in endothelial cells [49] and in mouse mesangial cells [50].
GCs lead to the activation and nuclear translocation of the GR, where it regulates gene transcription through transactivation and transrepression [51]. Some of the genes affected by GCs have been shown to be involved in GC-induced apoptosis of lymphoma cells [27, 28]. Our present data suggest the involvement of TXNIP as one of the mediators of GC-induced apoptosis in beta cells and its potential contribution to the development of steroid diabetes. While both GR and p38 activation are required for TXNIP induction and beta cell death, future studies are required to map the pro-apoptotic and pro-survival pathways modified by Dex and/or TXNIP in islet beta cells.
Abbreviations
- Akt:
-
Protein kinase B
- ChREBP:
-
Carbohydrate response element binding protein
- Dex:
-
Dexamethasone
- GCs:
-
Glucocorticoids
- GR:
-
Glucocorticoid receptor
- MAPK:
-
Mitogen-activated protein kinase
- PDE:
-
Phosphodiesterase
- TXNIP:
-
Thioredoxin-interacting protein
- TRX:
-
Thioredoxin
- VDUP1:
-
Vitamin D3-upregulated protein 1
- VitD3:
-
1α,25-Dihydroxyvitamin D3
References
Huang W, Glass CK (2010) Nuclear receptors and inflammation control: molecular mechanisms and pathophysiological relevance. Arterioscler Thromb Vasc Biol 30:1542–1549
Saad MJ, Folli F, Kahn JA, Kahn CR (1993) Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J Clin Invest 92:2065–2072
van Raalte DH, Ouwens DM, Diamant M (2009) Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest 39:81–93
Rhodes CJ (2005) Type 2 diabetes—a matter of beta-cell life and death? Science 307:380–384
Khan A, Ostenson CG, Berggren PO, Efendic S (1992) Glucocorticoid increases glucose cycling and inhibits insulin release in pancreatic islets of ob/ob mice. Am J Physiol 263:E663–E666
Delaunay F, Khan A, Cintra A et al (1997) Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest 100:2094–2098
Lambillotte C, Gilon P, Henquin JC (1997) Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest 99:414–423
Larsson H, Ahren B (1999) Insulin resistant subjects lack islet adaptation to short-term dexamethasone-induced reduction in insulin sensitivity. Diabetologia 42:936–943
Jeong IK, Oh SH, Kim BJ et al (2001) The effects of dexamethasone on insulin release and biosynthesis are dependent on the dose and duration of treatment. Diabetes Res Clin Pract 51:163–171
van Raalte DH, Nofrate V, Bunck MC et al (2010) Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. European J Endocr/European Federation of Endocrine Societies 162:729–735
van Raalte DH, van Genugten RE, Linssen MM, Ouwens DM, Diamant M (2011) Glucagon-like peptide-1 receptor agonist treatment prevents glucocorticoid-induced glucose intolerance and islet-cell dysfunction in humans. Diabetes Care 34:412–417
Scharfmann R, Duvillie B, Stetsyuk V, Attali M, Filhoulaud G, Guillemain G (2008) Beta-cell development: the role of intercellular signals. Diabetes Obes Metab 10(Suppl 4):S195–S200
Blondeau B, Lesage J, Czernichow P, Dupouy JP, Breant B (2001) Glucocorticoids impair fetal beta-cell development in rats. Am J Physiol 281:E592–E599
Gesina E, Tronche F, Herrera P et al (2004) Dissecting the role of glucocorticoids on pancreas development. Diabetes 53:2322–2329
Goodman PA, Medina-Martinez O, Fernandez-Mejia C (1996) Identification of the human insulin negative regulatory element as a negative glucocorticoid response element. Mol Cell Endocrinol 120:139–146
Sharma S, Jhala US, Johnson T, Ferreri K, Leonard J, Montminy M (1997) Hormonal regulation of an islet-specific enhancer in the pancreatic homeobox gene STF-1. Mol Cell Biol 17:2598–2604
Zawalich WS, Tesz GJ, Yamazaki H, Zawalich KC, Philbrick W (2006) Dexamethasone suppresses phospholipase C activation and insulin secretion from isolated rat islets. Metabolism 55:35–42
Ranta F, Avram D, Berchtold S et al (2006) Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes 55:1380–1390
Chen KS, DeLuca HF (1994) Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta 1219:26–32
Nishinaka Y, Masutani H, Nakamura H, Yodoi J (2001) Regulatory roles of thioredoxin in oxidative stress-induced cellular responses. Redox Rep 6:289–295
Chutkow WA, Patwari P, Yoshioka J, Lee RT (2008) Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J Biol Chem 283:2397–2406
Shalev A (2008) Lack of TXNIP protects beta-cells against glucotoxicity. Biochem Soc Trans 36:963–965
Shaked M, Ketzinel-Gilad M, Ariav Y, Cerasi E, Kaiser N, Leibowitz G (2009) Insulin counteracts glucotoxic effects by suppressing thioredoxin-interacting protein production in INS-1E beta cells and in Psammomys obesus pancreatic islets. Diabetologia 52:636–644
Cha-Molstad H, Saxena G, Chen J, Shalev A (2009) Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J Biol Chem 284:16898–16905
Chen J, Hui ST, Couto FM et al (2008) Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J 22:3581–3594
Chen J, Fontes G, Saxena G, Poitout V, Shalev A (2010) Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid-induced ER stress-mediated beta-cell death. Diabetes 59:440–447
Sionov RV, Spokoini R, Kfir-Erenfeld S, Cohen O, Yefenof E (2008) Mechanisms regulating the susceptibility of hematopoietic malignancies to glucocorticoid-induced apoptosis. Adv Cancer Res 101:127–248
Kfir-Erenfeld S, Sionov RV, Spokoini R, Cohen O, Yefenof E (2010) Protein kinase networks regulating glucocorticoid-induced apoptosis of hematopoietic cancer cells: fundamental aspects and practical considerations. Leuk Lymphoma 51:1968–2005
Chen Z, Lopez-Ramos DA, Yoshihara E et al (2010) Thioredoxin-binding protein-2 (TBP-2/VDUP1/TXNIP) regulates T cell sensitivity to glucocorticoid during HTLV-I-induced transformation. Leukemia 25:440–448
Eldor R, Yeffet A, Baum K et al (2006) Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci USA 103:5072–5077
Brunstedt J, Nielsen JH (1981) Direct long-term effect of hydrocortisone on insulin and glucagon release from mouse pancreatic islets in tissue culture. Acta Endocrinol 96:498–504
Wang Z, Rong YP, Malone MH, Davis MC, Zhong F, Distelhorst CW (2006) Thioredoxin-interacting protein (txnip) is a glucocorticoid-regulated primary response gene involved in mediating glucocorticoid-induced apoptosis. Oncogene 25:1903–1913
Avram D, Ranta F, Hennige AM et al (2008) IGF-1 protects against dexamethasone-induced cell death in insulin secreting INS-1 cells independent of AKT/PKB phosphorylation. Cell Physiol Biochem 21:455–462
Davani B, Portwood N, Bryzgalova G et al (2004) Aged transgenic mice with increased glucocorticoid sensitivity in pancreatic beta-cells develop diabetes. Diabetes 53(Suppl 1):S51–S59
Billaudel B, Mathias PC, Sutter BC, Malaisse WJ (1984) Inhibition by corticosterone of calcium inflow and insulin release in rat pancreatic islets. J Endocrinol 100:227–233
Pierluissi J, Navas FO, Ashcroft SJ (1986) Effect of adrenal steroids on insulin release from cultured rat islets of Langerhans. Diabetologia 29:119–121
Gremlich S, Roduit R, Thorens B (1997) Dexamethasone induces posttranslational degradation of GLUT2 and inhibition of insulin secretion in isolated pancreatic beta cells. Comparison with the effects of fatty acids. J Biol Chem 272:3216–3222
Ullrich S, Berchtold S, Ranta F et al (2005) Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes 54:1090–1099
Zhang X, Yong W, Lv J et al (2009) Inhibition of forkhead box O1 protects pancreatic beta-cells against dexamethasone-induced dysfunction. Endocrinology 150:4065–4073
Shalev A, Pise-Masison CA, Radonovich M et al (2002) Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology 143:3695–3698
Rani S, Mehta JP, Barron N et al (2010) Decreasing Txnip mRNA and protein levels in pancreatic MIN6 cells reduces reactive oxygen species and restores glucose regulated insulin secretion. Cell Physiol Biochem 25:667–674
Hotta M, Tashiro F, Ikegami H et al (1998) Pancreatic beta cell-specific expression of thioredoxin, an antioxidative and antiapoptotic protein, prevents autoimmune and streptozotocin-induced diabetes. J Exp Med 188:1445–1451
Yamamoto M, Yamato E, Toyoda S et al (2008) Transgenic expression of antioxidant protein thioredoxin in pancreatic beta cells prevents progression of type 2 diabetes mellitus. Antioxid Redox Signal 10:43–49
Nishiyama A, Matsui M, Iwata S et al (1999) Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem 274:21645–21650
Butler LM, Zhou X, Xu WS et al (2002) The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci USA 99:11700–11705
Oka S, Masutani H, Liu W et al (2006) Thioredoxin-binding protein-2-like inducible membrane protein is a novel vitamin D3 and peroxisome proliferator-activated receptor (PPAR)gamma ligand target protein that regulates PPARgamma signaling. Endocrinology 147:733–743
Shao J, Qiao L, Friedman JE (2004) Prolactin, progesterone, and dexamethasone coordinately and adversely regulate glucokinase and cAMP/PDE cascades in MIN6 beta-cells. Am J Physiol 286:E304–E310
Shao W, Yu Z, Fantus IG, Jin T (2010) Cyclic AMP signaling stimulates proteasome degradation of thioredoxin interacting protein (TxNIP) in pancreatic beta-cells. Cell Signal 22:1240–1246
Li X, Rong Y, Zhang M et al (2009) Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem Biophys Res Commun 381:660–665
Ren Y, Shi Y, Wang Y et al (2010) p38 MAPK pathway is involved in high glucose-induced thioredoxin interacting protein induction in mouse mesangial cells. FEBS Lett 584:3480–3485
Zanchi NE, Filho MA, Felitti V, Nicastro H, Lorenzeti FM, Lancha AH Jr (2010) Glucocorticoids: extensive physiological actions modulated through multiple mechanisms of gene regulation. J Cell Physiol 224:311–315
Acknowledgements
We are very grateful to L. Havin for the microarray analysis, A. Maklakov for her assistance, and to D. Sever and L. Amior for their technical help and very helpful discussions. Our sincere thanks to L. Piemonti and N. Rita, European Consortium for Islet Transplantation at the San Raffaele Hospital (Milan, Italy) for the supply of human islets. Many thanks to D. Sever for helping with graphic design.
Funding
This work was supported by grants from the European Union (STREP Savebeta, contract no. 036903) in the Framework Programme 6 and by the Israel Science Foundation (grant no. 936-11).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
ER, AT and RS performed research, analysed data, contributed to the conception and design, and analysis and interpretation of data described in this study. RS supervised and all authors critically revised the manuscript. DM directed the study, the conception of the experiments, interpretation of the data and writing the paper. All the authors approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Reich and A. Tamary contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
PDF 178 kb
Rights and permissions
About this article
Cite this article
Reich, E., Tamary, A., Sionov, R.V. et al. Involvement of thioredoxin-interacting protein (TXNIP) in glucocorticoid-mediated beta cell death. Diabetologia 55, 1048–1057 (2012). https://doi.org/10.1007/s00125-011-2422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2422-z