Abstract
Aims/hypothesis
The aim of this study was to determine whether the mean size of fat cells in either visceral or subcutaneous adipose tissue has an impact on the metabolic and inflammatory profiles in morbid obesity.
Methods
In 80 morbidly obese women, mean visceral (omental) and subcutaneous fat cell sizes were related to in vivo markers of inflammation, glucose metabolism and lipid metabolism.
Results
Visceral, but not subcutaneous, adipocyte size was significantly associated with plasma apolipoprotein B, total cholesterol, LDL-cholesterol and triacylglycerols (p ranging from 0.002 to 0.015, partial r ranging from 0.3 to 0.4). Subcutaneous, but not visceral, adipocyte size was significantly associated with plasma insulin and glucose, insulin-induced glucose disposal and insulin sensitivity (p ranging from 0.002 to 0.005, partial r ranging from −0.34 to 0.35). The associations were independent of age, BMI, body fat mass or body fat distribution. Adipose tissue hyperplasia (i.e. many small adipocytes) in both regions was significantly associated with better glucose, insulin and lipid profiles compared with adipose hypertrophy (i.e. few large adipocytes) in any or both regions (p ranging from <0.0001 to 0.04). Circulating inflammatory markers were not associated with fat cell size or corresponding gene expression in the fat cell regions examined.
Conclusions/interpretation
In morbidly obese women region-specific variations in mean adipocyte size are associated with metabolic complications but not systemic or adipose inflammation. Large fat cells in the visceral region are linked to dyslipidaemia, whereas large subcutaneous adipocytes are important for glucose and insulin abnormalities. Hyperplasia (many small adipocytes) in both adipose regions may be protective against lipid as well as glucose/insulin abnormalities in obesity.
Similar content being viewed by others
Introduction
Obesity is associated with generalised low-grade inflammation, dyslipidaemia, impaired glucose metabolism and insulin resistance, which are factors underlying obesity-mediated type 2 diabetes and cardiovascular disease [1]. The amount and distribution of adipose tissue is important for these obesity complications. A central accumulation of fat, in particular visceral fat, has a stronger relationship with metabolic and inflammatory markers than peripheral fat accumulation [1–7].
Another factor that may link adipose tissue to diabetic and atherogenic complications of obesity is its morphology. In adults, human adipose tissue is very dynamic. As much as 10% of the fat cells die and are renewed every year [8]. The fat mass can grow by increasing the size, rather than the number, of fat cells (hypertrophy) or by making many smaller fat cells (hyperplasia). Individuals with hypertrophy have slower adipogenesis and cell death than those with hyperplasia [9]. Although it is well established that the average fat cell volume increases with increasing body fat mass, there is a large interindividual variation in fat cell size irrespective of the absolute amount of adipose tissue. Obese individuals can have many smaller fat cells (i.e. hyperplasia) or fewer, but larger, adipocytes (i.e. hypertrophy), as reviewed by Hirsch and Batchelor [10].
Increased subcutaneous fat cell volume predicts impaired glucose metabolism, hyperinsulinaemia and insulin resistance [11–13]. Individuals with large fat cells are more prone to develop type 2 diabetes than those with small cells [14, 15]. An association of subcutaneous adipose hypertrophy (few large fat cells) with decreased insulin sensitivity has been determined in a large population-based sample [9]. So far, the effects of fat cell size on glucose/insulin variables have been examined only in subcutaneous adipose tissue. The role of visceral fat is unknown. This regional aspect is clinically relevant as visceral fat is considered to be more ‘diabetogenic’ than subcutaneous adipose tissue according to reviews [2–6]. The influence of fat cell size on circulating lipid levels has been examined in one study [16]. The role of adipocyte size with respect to inflammation is not known.
We examined the impact of mean fat cell size in visceral and subcutaneous adipose tissues on the metabolic and inflammatory profiles of morbidly obese women. The combined regional influence on insulin, glucose, inflammation and lipid variables was examined. Our primary hypothesis was that fat cell size is linked to metabolic and inflammatory markers differently in the subcutaneous compared with the visceral region. Our secondary hypothesis was that having adipose hyperplasia (many small fat cells) in both adipose compartments is associated with a more benign metabolic profile than having hypertrophy (few large fat cells) in any or both adipose regions.
Methods
Individuals
The study included 80 women who were scheduled for gastric bypass surgery for morbid obesity (BMI ≥ 35 kg/m2). Age, waist circumference and BMI were 21–64 years, 107–155 cm and 35–62 kg/m2, respectively. Average total body fat and distribution of android/gynoid fat were 42–67% and 0.88–1.32, respectively. There were 21 postmenopausal women. None was on hormone replacement therapy. Seven women were receiving treatment for type 2 diabetes with diet plus sulfonylurea or biguanide. None had impaired fasting glucose. Three women were using statins for dyslipidaemia. The study was explained in detail to each participant and informed written consent was obtained. The study was approved by the local committee on ethics.
After an overnight fast, total body fat and fat distribution in the central (android) and the peripheral (gynoid) areas were determined by dual-energy X-ray absorptiometry (DEXA) using a GE-Lunar iDXA (GE Healthcare, Madison, WI, USA) and associated software (enCore 10.20), as described by Lee et al. [17]. A venous blood sample was obtained for measurement of glucose, insulin, total cholesterol, HDL-cholesterol, triacylglycerols and insulin, and insulin and glucose values were used to calculate an insulin sensitivity index (HOMA), as described by Wahrenberg et al. [18]. LDL-cholesterol was calculated according to the Friedewald formula [19]. All participants had plasma triacylglycerol <4.5 mmol/l (the upper limit for estimates with the formula). Apolipoprotein B and apolipoprotein A-1 in plasma were measured by the hospital’s accredited routine chemistry laboratory. Serum IL-6, TNF-α, fibrinogen, C-reactive protein and monocyte chemotactic protein-1 were determined using commercially available ELISA kits (R&D Systems, Abingdon, UK). Height, weight and waist circumference were measured and a subcutaneous abdominal fat biopsy was obtained as described by Lofgren et al. [20]. Subsequently, the patients were subjected to a hyperinsulinaemic–euglycaemic clamp as described by Hagstrom-Toft et al. [21]. In brief, they were given a bolus dose of insulin intravenously (1.6 U/m2 body surface area; Actrapid, Novo Nordisk, Copenhagen, Denmark) and then an insulin infusion was delivered intravenously (0.12 U/m2 min) for 120 min. Blood glucose values were measured in duplicate every fifth minute (HemoCue, Ängelholm, Sweden) and euglycaemia was maintained by a variable intravenous infusion of glucose (50 mg/ml). The measurements from the last 60 min of the clamp were used for calculations of glucose-disposal rate. During this period, the insulin levels (mean ± SD) were 1,669 ± 528 pmol/l. Visceral adipose tissue from the major omentum was obtained on a separate occasion no more than 3 weeks later—specifically, at the beginning of bariatric surgery. Body weight was essentially the same at both examinations.
Determination of adipocyte size
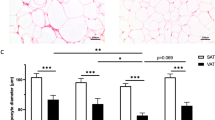
Isolated fat cells were obtained from the adipose tissue sample (0.5–1.0 g) for the determination of fat cell volume, as described by Lofgren et al. [20]. In brief, adipocytes were isolated from the stromal–vascular compartment by collagenase digestion and the diameters of 100 fat cells were measured with a calibrated light microscope. The mean volume of the isolated adipocytes was calculated using published formulae [22]. In the whole cohort the fat cell diameter varied between 100 and 290 μm in subcutaneous adipose tissue and between 62 and 177 μm in visceral adipose tissue.
Determination of adipose tissue morphology
The relationship between mean adipocyte volume and total body fat determines adipocyte morphology [8, 9]. Briefly, the relationship is described by the following equation: \( \left( {V = a \times m} \right)/\left( {{1} + b \times m} \right) \), where V is the average adipocyte volume (pl), m is the amount of body fat (kg), and a and b are variables that can be estimated by fitting the formula to patient data. We used our previously published data regarding this relationship between subcutaneous adipocyte volume and body fat in 557 women [9] to estimate a sc to 40.1 pl/kg and b sc to 0.030/kg for the subcutaneous depot. Similarly, we used another previously published data set [8] from the visceral depot in 84 women to estimate the corresponding variables a vis (17.7 pl/kg) and b vis (0.0150/kg) for the visceral depot. The differences between observed adipocyte volume in the present investigation and the expected volume (as obtained from the curve fit) at the corresponding level of total body fat mass were calculated separately in both depots for each woman, who were classified as having either hyperplasia (negative deviation) or hypertrophy (positive deviation) relative to the estimated average value for body fat. The morphology values are quantitative: a larger absolute positive or negative value reflects more pronounced hypertrophy or hyperplasia, respectively, compared with a small value [9]. The data generated previously were calculated using a formula-derived measure of body fat mass [9]. Therefore, we also determined adipose morphology using only the study cohort. The relationship between body fat (by DEXA) and fat cell volume was determined by linear regression and the morphology value was calculated as described above.
Gene expression
mRNA for IL6 and TNF-α (also known as TNF) was quantified in adipose tissue exactly as described by Ryden et al. [23]. In brief, total RNA was extracted from 300 mg adipose tissue using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The Agilent 2100 Bioanalyzer (Agilent Technologies, Kista, Sweden) was used to make cDNA using the Omniscript RT Kit (Qiagen) and random hexamer primers (Invitrogen, Taastrup, Denmark). cDNA synthesis was performed simultaneously for all patients using the same mix of primers and kit, but omental and subcutaneous samples were run separately. In a final volume of 25 μl, 5 ng of cDNA was mixed with ×2 SYBR Green PCR Master Mix (Bio-Rad Laboratories, Hercules, CA, USA) and primers (Invitrogen). The amplicon gave a single mRNA species, which was analysed for IL6 (NM_000600.2) and TNF-α (NM_000594.2). Quantitative real-time PCR was performed in an iCycler iQ (Bio-Rad Laboratories) and was quantified using TaqMan kits (Applied Biosystems, Foster City, CA, USA) for Hs0017413_ml (IL6) and Hs00174128_ml (TNF-α). Expression of mRNA was normalised to the 18S internal control by using a comparative C t method.
Statistical analysis
Values are mean ± SD and are compared by simple or multiple regression analysis, χ 2 test, paired t test and ANOVA, with Fisher’s protected least significant difference (PLSD) post-hoc test when appropriate. When indicated, some values were log10 transformed before comparison to improve normalisation. Power of test for the null hypothesis was performed using Sample Power (SPSS, Chicago, IL, USA). For multiple regression analysis, the model included two covariates (visceral and subcutaneous), which would yield an r 2 value of 0.150. The power analysis focused on the increment of the minor over the major covariate to detect a difference in correlation of values of r 2 of 0.05. With the given sample size of 80 and alpha set at 0.05, we had a statistical power of 89%. For ANOVA, we tested the same metabolic variables as tested with multiple regression analysis, using overall mean and SD for each of the variables. We could detect a 20% or larger difference between combined hyperplasia and the two other groups at p < 0.05 with 85% or better statistical power.
Results
We first examined the relationships between metabolic and inflammatory variables and fat cell size by univariate linear regression analysis (Table 1). Apolipoprotein A-1, HDL-cholesterol and inflammation variables did not correlate significantly with fat cell size in any region. Subcutaneous fat cell volume correlated significantly with plasma apolipoprotein B, triacylglycerols, glucose and insulin as well as with insulin-induced glucose disposal. Visceral adipocyte volume correlated significantly with plasma total cholesterol, LDL-cholesterol, triacylglycerols, apolipoprotein B, insulin and insulin-induced glucose disposal. Insulin levels during the last hour of the clamp did not correlate with adipocyte volume. Visceral and subcutaneous fat cell size correlated positively with each other (r = 0.41; p < 0.0001). All subsequent examinations focused on the significant associations shown in Table 1.
To test our primary hypothesis, we investigated the relative contributions of visceral and subcutaneous adipocyte volume to the variation in the above-mentioned metabolic variables (Table 2). Those showing significant results in univariate regression were correlated with the two adipocyte regressors together in a multiple regression analysis. Similar data were obtained if we instead used stepwise regression (results not shown). Visceral, but not subcutaneous, fat cell volume contributed significantly to the variations in plasma total cholesterol, LDL-cholesterol, apolipoprotein B and triacylglycerols. However, subcutaneous, but not visceral, fat cell size contributed significantly to the variations in insulin-induced glucose disposal rate, plasma glucose and insulin. Adjustment for age, BMI, the amount of android, gynoid or total body fat or regional fat distribution in the regression model did not change the outcome in any substantial way. The same was true if the ten women receiving treatment for type 2 diabetes or dyslipidaemia were excluded or not.
The significant p values in Table 2 were corrected for multiple comparison according to Bonferroni by multiplying them with the number of examined dependent variables (i.e. ×7). All p values except for apolipoprotein B vs visceral fat cell volume remained statistically significant (p < 0.05).
Insulin sensitivity was also indirectly determined from insulin and glucose values as HOMA index. Multiple regression showed that subcutaneous fat cell size (partial r = 0.34), but not visceral fat cell size (partial r = 0.07), contributed significantly (p = 0.004) to variations in the HOMA index. Insulin-induced glucose disposal and HOMA index were strongly correlated (r = −0.62; p < 0.001).
From the distribution of individual values above or below the previously established curvilinear relationship between fat mass and fat cell volume, the morphology value of subcutaneous (Electronic supplementary material [ESM] Fig. 1a) and visceral (ESM Fig. 1b) adipose tissue could be determined. When these data were categorised into hyperplasia or hypertrophy, there was no important difference in the distribution of these categories between the adipose regions (p = 0.12 by χ 2 test). We also calculated the morphology values using the DEXA-derived values for body fat mass and using solely the values from the present cohort (ESM Fig. 1c, d). When the values obtained by the different methods were compared by simple regression almost identical results were obtained (r = 0.99 and slope 0.99 for the subcutaneous region and r = 0.97 and slope 0.98 for the visceral region). The average fat cell volume was larger in the subcutaneous region (989 ± 174 pl) than in the visceral region (631 ± 184 pl; p < 0.0001).
To test our secondary hypothesis, women were categorised according to their adipose morphology. Thirteen women had hyperplasia in both regions (combined hyperplasia), 36 women had hypertrophy in both regions (combined hypertrophy), 23 women had visceral hyperplasia but subcutaneous hypertrophy and eight women had visceral hypertrophy but subcutaneous hyperplasia. In order to increase the statistical power the last two groups were combined (heterogeneous morphology). The clinical characteristics of the three groups are presented in Table 3. The combined hyperplasia group had much smaller fat cells than the heterogeneous or combined hypertrophy group (p < 0.001). Age, BMI, total body fat, body fat distribution and waist circumference were not significantly different between groups. However, HOMA index and all metabolic factors that are listed in Table 2 differed significantly between groups. For all variables, combined hyperplasia showed the most benign values (p < 0.05 by the sign test) and these were always significantly different from the combined hypertrophy values. For plasma insulin, HOMA index and insulin-induced glucose disposal, the combined hyperplasia group also had significantly more benign values than the heterogeneous group. The distribution of individuals with diabetes or dyslipidaemia differed between groups (p < 0.05); none had combined hyperplasia.
Expression of mRNA relative to the reported gene 18S was measured for two major inflammatory genes, namely IL6 and TNF-α (also known as TNF). IL6 mRNA in omental adipose tissue showed a borderline positive correlation with omental fat cell size (r = 0.21; p = 0.062). No other correlation between gene expression and fat cell size was recorded (r = 0.03–0.17; p = 0.13–0.76). Expression of the mRNA of the inflammatory genes did not correlate with their corresponding circulating protein levels (values not shown).
The influences of visceral and subcutaneous fat cell volumes on body shape variables were compared using multiple regression analysis. None of the volumes showed a significant correlation with body fat distribution or waist-to-hip ratio when considered together (β coefficient from −0.002 to 0.23; p value from 0.06 to 0.98). Subcutaneous, but not visceral, fat cell volume correlated with waist circumference (β coefficient 0.31 and −0.05, respectively; p value 0.01 and 0.65, respectively). The subcutaneous correlation was independent of age and body fat content (β coefficients and p values for subcutaneous fat cell volume 0.23–0.32 and 0.01–0.04, respectively). However, the effect of subcutaneous fat cell volume disappeared after correction for BMI.
Discussion
Except for one previous study examining plasma lipids [16], information on the role of fat cell size in visceral adipose tissue for diabetic and atherogenic obesity complications has not been published. Our findings clearly indicate quite a different impact of subcutaneous and visceral fat cell sizes on lipid and glucose/insulin profiles among obese women. A power analysis and adjustments for multiple testing indicated that our sample size had sufficient statistical power to reveal clinically significant differences between groups.
The impact of fat cell size on the circulatory inflammatory profile has not been examined previously. In our obese women there was no relationship between adipose cellularity and inflammatory markers. This was surprising as obese adipose tissue is in an inflammatory state, resulting in the release of a number of inflammatory agents into the circulation, as reviewed by Gustafson et al. [24]. Although adipose tissue may be inflamed in obesity, its morphology seems not to be involved in the release of inflammatory markers to the circulation. We also investigated the expression of genes encoding two of the most important inflammatory factors in adipose tissue, namely IL-6 and TNF-α [24]. There was, however, no significant relationship between fat cell size and mRNA for IL-6 or TNF-α, further suggesting a less important role of fat cell size for inflammation in obesity.
Previous studies demonstrated distinct pools of very small fat cells in subcutaneous and visceral human adipose tissue [25–28]. The diameter of these cells is in the range 20–50 μm [27, 28]. Surprisingly, there is an increase in the fraction of very small subcutaneous fat cells in individuals with insulin resistance or adipose inflammation [26, 28]. This is in apparent contrast to functional studies of small and large subcutaneous fat cells obtained from the same individuals, demonstrating that small cells have decreased production and secretion of inflammatory factors [29, 30] and are more insulin responsive than large adipocytes [31]. Thus, the true biological roles of the small and large fat cells within an individual need to be better defined. Although we found no very small cells in subcutaneous or visceral fat cells of our patients, our experimental process may have caused us to overlook the pool of very small fat cells. As discussed by Sparks et al. [32], the current use of isolated fat cells obtained by collagenase isolation of small amounts of adipose tissue might result in the loss of most of the very small cells. In the previous studies, osmium tetroxide fixation of adipocytes was used, which allows fat cells of all sizes to be retained within the adipose specimens [25–28]. Therefore, the possible contribution of very small fat cells to our findings is not known for the moment. However, they are representative of the pool of adipocytes with large fat cells. With regard to large-sized adipocytes, our data agree with previous findings showing no correlation between mRNA expression of inflammatory genes and mean cell diameter of the pool of large subcutaneous adipocytes [28].
In our obese individuals, visceral adipose tissue had a much stronger impact on the plasma lipid profile than had subcutaneous adipose tissue. This confirms the findings of a previous study on obese individuals by Garaulet et al. [16]. In the visceral region, enlarged fat cell size was quantitatively associated with elevated plasma levels of total cholesterol, LDL-cholesterol, triacylglycerols and apolipoprotein B. This relationship was not observed for subcutaneous adipose tissue when examined together with the visceral fat depot. Furthermore, the findings were independent of age, BMI, total body fat and body fat distribution. These results underscore a role for enlarged visceral adipocytes for atherogenic obesity complications.
The impact of visceral fat cell size on insulin/glucose variables has not been published previously. These variables were much more strongly related to subcutaneous adipocyte size than visceral adipocyte size when the two regions were examined together. Thus, obese women with large subcutaneous fat cells had higher HOMA index, plasma insulin and glucose levels and lower insulin-induced glucose disposal than those with small subcutaneous fat cells. Again, the findings were independent of age, BMI, total body fat and body fat distribution. When compared with the subcutaneous region, the size of visceral fat cells had no relationship with the insulin or glucose variables. This illuminates a role of large subcutaneous fat cells for the diabetogenic effect of obesity.
Presently, we can only speculate about the mechanisms making subcutaneous fat cell size more important for altered glucose metabolism and the visceral fat cell size important for dyslipidaemia. The subcutaneous fat depot is the largest adipose region [24] and may quantitatively play the leading role for metabolic flexibility. This is the capacity for muscle to shift reliance between glucose and lipids in response to insulin, which is impaired in individuals with large subcutaneous fat cells [32]. In quantitative terms, more glucose is taken up by subcutaneous adipose tissue than by visceral adipose tissue following insulin infusion and this regional difference is enhanced following treatment with insulin sensitisers (biguanides or glitazones) [33, 34]. With regard to plasma lipids, visceral adipose tissue is drained by the portal vein directly into the liver where the production of circulating lipids and lipoprotein takes place. The contribution of visceral fatty acids to VLDL-triacylglycerols is increased in insulin-resistant individuals [35]. A low capacity of large adipocytes to fill up with more lipids could play a role in dyslipidaemia [36]. Human adipose tissue is important for the production and storage of cholesterol as reviewed by Miettinen and Tilvis [37]. It is possible that fat cell size in subcutaneous and visceral adipose tissues has a region-specific impact on the above-mentioned lipid metabolism processes so that the visceral region has the strongest link to the altered triacylglycerol/cholesterol pattern in the plasma of obese women. Finally, visceral and subcutaneous fat cells may be of different origin and have specific functions. We confirmed the findings of previous studies [6], that subcutaneous fat cells are larger than visceral ones. In vitro studies show that human subcutaneous and visceral adipocytes have qualitatively different responses to hormone stimulation [38, 39].
It is apparent that general adipose hyperplasia—that is, many smaller fat cells in both the subcutaneous and the visceral areas—is less pernicious as regards the metabolic complications of obesity. Women with combined hyperplasia had a more benign glucose, insulin and lipid metabolic profile than those with hypertrophy in either or both regions (few but larger fat cells). Furthermore, none of the women with general adipose hyperplasia had overt diabetes or dyslipidaemia. Methodological investigation showed that the assignment of women to the hyperplasia or hypertrophy groups was valid.
We examined only women with morbid obesity. Thus, the impact of regional fat cell size in men and in women with less severe obesity may be different.
In conclusion, in addition to adipose mass and distribution, mean fat cell size is associated with metabolic complications but not with systemic or adipose inflammation in morbid obesity. This influence is region specific. Large visceral fat cells are more strongly linked to dyslipidaemia, whereas large subcutaneous fat cells correlate with impaired glucose metabolism, hyperinsulinaemia and insulin resistance. Obese women with many, but smaller, fat cells in both adipose regions may be protected from metabolic complications. These conclusions are made from a cross-sectional study on morbidly obese women. It is not known whether the findings will hold true for obese men, for women with mild obesity or after weight loss, which in itself ameliorates obesity complications. For ethical reasons, it is not possible to perform multiple visceral adipose tissue biopsies in prospective studies following bariatric surgery. Furthermore, the role of very small fat cells, and relevance to the present findings, remains to be established.
Abbreviations
- DEXA:
-
Dual-energy X-ray absorptiometry
- PLSD:
-
Protected least significant difference
References
Grundy SM (2004) Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89:2595–2600
Despres JP, Lemieux I, Bergeron J et al (2008) Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 28:1039–1049
Garg A (2004) Regional adiposity and insulin resistance. J Clin Endocrinol Metab 89:4206–4210
Hamdy O, Porramatikul S, Al-Ozairi E (2006) Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diab Rev 2:367–373
Howard BV, Ruotolo G, Robbins DC (2003) Obesity and dyslipidemia. Endocrinol Metab Clin North Am 32:855–867
Wajchenberg BL (2000) Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21:697–738
Zeyda M, Stulnig TM (2009) Obesity, inflammation, and insulin resistance—a mini-review. Gerontology 55:379–386
Spalding KL, Arner E, Westermark PO et al (2008) Dynamics of fat cell turnover in humans. Nature 453:783–787
Arner E, Westermark PO, Spalding KL et al (2010) Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 59:105–109
Hirsch J, Batchelor B (1976) Adipose tissue cellularity in human obesity. Clin Endocrinol Metab 5:299–311
Krotkiewski M, Sjostrom L, Bjorntorp P, Smith U (1975) Regional adipose tissue cellularity in relation to metabolism in young and middle-aged women. Metab Clin Exp 24:703–710
Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson JW (2007) Fat cell enlargement is an independent marker of insulin resistance and ‘hyperleptinaemia’. Diabetologia 50:625–633
Stern JS, Batchelor BR, Hollander N, Cohn CK, Hirsch J (1972) Adipose-cell size and immunoreactive insulin levels in obese and normal-weight adults. Lancet 2:948–951
Lonn M, Mehlig K, Bengtsson C, Lissner L (2010) Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J 24:326–331
Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE (2000) Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 43:1498–1506
Garaulet M, Perez-Llamas F, Zamora S, Tebar FJ (2002) Interrelationship between serum lipid profile, serum hormones and other components of the metabolic syndrome. J Physiol Biochem 58:151–160
Lee K, Lee S, Kim YJ, Kim YJ (2008) Waist circumference, dual-energy X-ray absortiometrically measured abdominal adiposity, and computed tomographically derived intra-abdominal fat area on detecting metabolic risk factors in obese women. Nutrition 24:625–631
Wahrenberg H, Hertel K, Leijonhufvud BM, Persson LG, Toft E, Arner P (2005) Use of waist circumference to predict insulin resistance: retrospective study. BMJ 330:1363–1364
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Lofgren P, Hoffstedt J, Ryden M et al (2002) Major gender differences in the lipolytic capacity of abdominal subcutaneous fat cells in obesity observed before and after long-term weight reduction. J Clin Endocrinol Metab 87:764–771
Hagstrom-Toft E, Thorne A, Reynisdottir S et al (2001) Evidence for a major role of skeletal muscle lipolysis in the regulation of lipid oxidation during caloric restriction in vivo. Diabetes 50:1604–1611
Hirsch J, Gallian E (1968) Methods for the determination of adipose cell size in man and animals. J Lipid Res 9:110–119
Ryden M, Agustsson T, Laurencikiene J et al (2008) Lipolysis—not inflammation, cell death, or lipogenesis—is involved in adipose tissue loss in cancer cachexia. Cancer 113:1695–1704
Gustafson B, Hammarstedt A, Andersson CX, Smith U (2007) Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol 27:2276–2283
Julien P, Despres JP, Angel A (1989) Scanning electron microscopy of very small fat cells and mature fat cells in human obesity. J Lipid Res 30:293–299
McLaughlin T, Sherman A, Tsao P et al (2007) Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 50:1707–1715
Liu A, McLaughlin T, Liu T et al (2009) Differential intra-abdominal adipose tissue profiling in obese, insulin-resistant women. Obes Surg 19:1564–1573
McLaughlin T, Deng A, Yee G et al (2010) Inflammation in subcutaneous adipose tissue: relationship to adipose cell size. Diabetologia 53:369–377
Jernas M, Palming J, Sjoholm K et al (2006) Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J 20:1540–1542
Skurk T, Alberti-Huber C, Herder C, Hauner H (2007) Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92:1023–1033
Franck N, Stenkula KG, Ost A, Lindstrom T, Stralfors P, Nystrom FH (2007) Insulin-induced GLUT4 translocation to the plasma membrane is blunted in large compared with small primary fat cells isolated from the same individual. Diabetologia 50:1716–1722
Sparks LM, Ukropcova B, Smith J et al (2009) Relation of adipose tissue to metabolic flexibility. Diab Res Clin Pract 83:32–43
Virtanen KA, Hallsten K, Parkkola R et al (2003) Differential effects of rosiglitazone and metformin on adipose tissue distribution and glucose uptake in type 2 diabetic subjects. Diabetes 52:283–290
Virtanen KA, Lonnroth P, Parkkola R et al (2002) Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab 87:3902–3910
Hodson L, Bickerton AS, McQuaid SE et al (2007) The contribution of splanchnic fat to VLDL triglyceride is greater in insulin-resistant than insulin-sensitive men and women: studies in the postprandial state. Diabetes 56:2433–2441
Smith J, Al-Amri M, Dorairaj P, Sniderman A (2006) The adipocyte life cycle hypothesis. Clin Sci 110:1–9
Miettinen TA, Tilvis RS (1981) Cholesterol synthesis and storage in adipose tissue. Int J Obes 5:613–618
Dicker A, Ryden M, Naslund E et al (2004) Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia 47:420–428
van Harmelen V, Eriksson A, Astrom G et al (2008) Vascular peptide endothelin-1 links fat accumulation with alterations of visceral adipocyte lipolysis. Diabetes 57:378–386
Acknowledgements
We are grateful for the excellent technical assistance of B.-M. Leijonhufvud, K. Hertel, E. Sjölin and K. Wåhlén. This study was supported by grants from the Swedish Research Council, Swedish Diabetes Association, Swedish Heart and Lung Association, Diabetes programme at Karolinska Institutet and the Novo Nordisk Foundation. J. Hoffstedt, E. Arner, H. Wahrenberg, D. P. Andersson, V. Qvisth, P. Löfgren, M. Rydén, M. Palmér and E. Toft performed and/or supervised analyses and experiments. A. Thörne, M. Wirén and A. Thorell recruited and selected patients. P. Arner planned the study and wrote the first version of the paper. All authors contributed to further versions of the manuscript and approved the final one.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
Relationship between fat cell size and fat mass in subcutaneous adipose tissue (a, c) and visceral adipose tissue (b, d). The lines in a and b were obtained from previous examinations on large cohorts (grey dots). The values for body fat and fat cell volume in the currently studied cohort are indicated by black dots. The lines in c and d were obtained from examining only the present cohort. The morphology value is the difference between measured fat cell volume and the volume at the corresponding body fat mass indicated by the line. A measured volume above the volume predicted by the line gives a positive value and indicates hypertrophy. A value below the line gives a negative morphology value and indicates hyperplasia (PDF 118 kb)
Rights and permissions
About this article
Cite this article
Hoffstedt, J., Arner, E., Wahrenberg, H. et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 53, 2496–2503 (2010). https://doi.org/10.1007/s00125-010-1889-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-010-1889-3