Abstract
Aims/hypothesis
This study was designed to investigate the use and impact of a continuous glucose monitoring system (the FreeStyle Navigator) under home-use conditions in the self-management of type 1 diabetes.
Methods
A 20 day masked phase, when real-time data and alarms were not available, was compared with a subsequent 40 day unmasked phase for a number of specified measures of glycaemic variability. HbA1c (measured by DCA 2000) and a hypoglycaemia fear survey were recorded at the start and end of the study.
Results
The study included 48 patients with type 1 diabetes (mean age 35.7 ± 10.9, range 18–61 years; diabetes duration 17.0 ± 9.5 years). Two patients did not complete the study for personal reasons. Comparing masked (all 20 days) and unmasked (last 20 days) phases, the following reductions were seen: time outside euglycaemia from 11.0 to 9.5 h/day (p = 0.002); glucose SD from 3.5 to 3.2 mmol/l (p < 0.001); hyperglycaemic time (>10.0 mmol/l) from 10.3 to 8.9 h/day (p = 0.0035); mean amplitude of glycaemic excursions (peak to nadir) down by 10% (p < 0.001); high blood glucose index down by 18% (p = 0.0014); and glycaemic risk assessment diabetes equation score down by 12% (p = 0.0013). Hypoglycaemic time (<3.9 mmol/l) decreased from 0.70 to 0.64 h/day without statistical significance (p > 0.05). Mean HbA1c fell from 7.6 ± 1.1% at baseline to 7.1 ± 1.1% (p < 0.001). In the hypoglycaemia fear survey, the patients tended to take less snacks at night-time after wearing the sensor.
Conclusions/interpretation
Home use of a continuous glucose monitoring system has a positive effect on the self-management of diabetes. Thus, continuous glucose monitoring may be a useful tool to decrease glycaemic variability.
Similar content being viewed by others
Introduction
The increased availability of continuous glucose sensors is likely to have a significant impact on diabetes therapy and education in the future. While a recent meta-analysis of retrospective first generation Holter-type sensors did not show advantages compared with self-monitoring of blood glucose levels [1], this may be different with current real-time sensors [2]. The FreeStyle Navigator continuous glucose monitoring (CGM) system (Abbott Diabetes Care, Alameda, CA, USA) is a CGM device that is based on wired enzyme technology and continually measures interstitial fluid glucose levels [3]. Recently, a major US trial of 322 adults and children wearing one of the three different commercially available real-time sensors [4] showed that CGM can be associated with improved glycaemic control. Particularly their finding that no effect was discernible in the age group below 25 years in an intention-to-treat analysis has lead to controversial discussion. Detailed analyses of the effects of the sensors on glycaemic variability are ongoing.
The importance of glycaemic variability for various outcomes in diabetes [5], but also in critically ill patients [6] is receiving more and more attention. In patients with type 2 diabetes a significant association has been reported between the mean amplitude of glycaemic excursions (MAGE), an established variable for glycaemic variability [7] and urinary 8-iso-prostaglandin F2 alpha, a variable related to superoxide overproduction and subsequent development of later complications [8].
While variability of glucose as a cause of complications is controversial, it is intuitively significant as a quality of life issue. Fluctuating blood glucose levels have been shown to be associated with the behavioural changes frequently reported by parents in their diabetic children [9]. Hypoglycaemia fear is one of the leading obstacles to better glycaemic control [10]. An improvement in the rate of hypoglycaemia alone through CGM would constitute a significant benefit in itself [11]. This suggests that different therapeutic strategies now in use [12] should be evaluated for their potential to minimise glycaemic excursions, as well as their ability to lower HbA1c. Treatment options to decrease glycaemic variability are not restricted to type 1 diabetes [13]. Therefore, wider use of real-time CGM in clinical practice would provide the required monitoring tool to minimise glycaemic variability [14, 15].
This first European study using a CGM system based on wired enzyme technology aims to evaluate in the home environment changes in glycaemic excursions and glycaemic variability in patients with diabetes. In order not to interfere with any other aspects of their ongoing treatment, the patients received no sensor-based treatment algorithms or external review of the glucose profiles during the 60 day trial. The patients served as their own control by comparing a phase with masked and unmasked sensor use. The effect of sensor use on different variables of glycaemic variability and hypoglycaemia fear was analysed.
Methods
Patients and study schedule
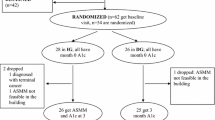
This 60 day study of continuous sensor wear (arm or abdomen) was conducted in the home environment in adult patients with type 1 diabetes. The patients performed sensor insertions and calibrations. A total of 48 participants were enrolled in the study (Table 1), of whom 39 were using insulin pumps (81.3%). Enrolled study participants met the following eligibility criteria: type 1 diabetes mellitus diagnosis at least 1 year prior to study enrolment; at least 18 years of age; and written informed consent.
Upon enrolment in the study, baseline review of participant status included documentation of participant demographics, current diabetes management and HbA1c (measured with DCA 2000 [Bayer, Leverkusen, Germany]). A fear of hypoglycaemia questionnaire [16, 17] was completed at baseline and after 60 days. The study was approved by the responsible ethics committees.
The first sensor was inserted at the clinic site. After 5 days of wear, participants returned to the site to remove this sensor and insert the second under supervision. During Phase 1 (duration 20 days), participants were masked to the display of the CGM data. For the masked phase, patients were instructed to make all treatment decisions based on capillary blood glucose tests only. At the end of Phase 1, participants returned to the clinic to have their CGM device unmasked, enabling the display of continuous glucose readings and activation of low- and high-glucose alarms. In the unmasked phase, participants were instructed to make treatment decisions based on CGM device data, except during hypoglycaemia or rapidly changing glucose values when confirmatory capillary blood glucose tests were to be performed prior to self-management decisions. Throughout the masked and unmasked phases of the study, glucose values from the interstitial glucose sensor and from the integrated FreeStyle blood glucose meter were stored electronically in the memory log of the CGM receiver.
Calculation of glucose excursions
An excursion is a period of time during which the participant's glucose profile is entirely within one status, either hypoglycaemic (<3.9 mmol/l), euglycaemic or hyperglycaemic (>10.0 mmol/l). Excursions consist of a group of data points, continuous in time, with time gaps less than or equal to 60 min. If a gap exceeds 60 min the excursion is considered finished and a new excursion begins at the next data point. Excursions must contain at least two data points and must be at least 10 min long in duration to be included in the analysis. Continuous glucose readings are stored in the receiver memory every 10 min. Therefore an excursion of 10 min will have at least two data points beyond the hypo or hyperglycaemic boundary.
Sample size validation
The primary endpoint for the study was to evaluate improvement of hypo- and hyperglycaemic excursions outside the range 3.9 to 10.0 mmol/l. The null and alternative hypotheses were as follows:
where the difference (d) was evaluated as follows for each participant: d = unmasked–masked. The results for each participant were then averaged to obtain \(\overline{d} \) for the study. In a previous study [18], the excursions in the masked vs unmasked phases were reduced by 10% with standard deviation of 23% (n = 122 participants). Using this standard deviation and a sample size of 40 participants, a difference in time (as percentage of masked mean), with hypo- and hyperglycaemic excursions of at least 10 min between the last 40 days of participant participation in Phase 2 compared with the initial 20-day participation in Phase 1, of 7.1% or greater would be significant. This would be calculated as:
where 1.96 is the value of the inverse normal function needed to give a 95% significance level. Sample size estimation was performed using SAS software (SAS Institute, Cary, NC, USA).
Results
A total of 46 participants recorded CGM data in the masked and unmasked study phases (Table 1). Two patients did not complete the study for personal reasons. These participants are not included in the overall glycaemic analysis. Two adverse events occurred during the study: an adhesive reaction and bleeding/pain on sensor insertion. No serious adverse events were reported.
The primary effectiveness endpoint, the proportion of time that glucose values were outside the acceptable range (3.9–10.0 mmol/l), was reduced by 13.4% (Table 2). An equivalent measure to proportion of time is hours per day. The mean number of hours per day spent outside the range 3.9–10.0 mmol/l was reduced by 13.4% from 11.0 h/day in the masked phase to 9.5 h/day in the last 20 study days (unmasked) (Table 2).
Regarding secondary effectiveness endpoints, mean HbA1c was reduced from 7.6 at baseline to 7.1% (p = 0.0001) (Fig. 1a). Nearly all current measures of glycaemic variability were reduced in the last 20 study days (unmasked phase) compared with the masked phase, the reductions being independent of baseline HbA1c. Standard deviation (Fig. 1b), MAGE [7], high blood glucose index (HBGI) [19] and glycaemic risk assessment diabetes equation score (GRADE) [20] were reduced, but not the lability index [20] or the low blood glucose index (LBGI) [19] (Fig. 2).
Improvement of HbA1c (a) and glycaemic variability (expressed as glucose SD) (b) in relation to HbA1c level at baseline. HbA1c improved significantly by −0.45 (95% CI for difference −0.66, −0.25; p = 0.0001). A notable reduction of glycaemic variability was seen in nearly all patients and was independent of baseline HbA1c
Changes in variables of glycaemic variability between the masked and the last 20 days of the unmasked phase. decr, MAGE peak to madir events; eu, euglycaemic, glucose ≥3.9 and ≤10 mmol; hyper, hyperglycaemic >10 mmol/l; hypo, hypoglycaemic <70 mmol/l; incr, MAGE nadir to peak events. *p < 0.05, **p < 0.01, ***p < 0.001
While the mean number of hours per day >10.0 mmol/l was reduced by 13.7% from 10.3 h/day in the masked phase to 8.9 h/day in the last 20 study days, no statistically significant differences were observed in the number of hours per day below 3.9 mmol/l or the number of hypoglycaemic excursions per day (Table 2). The reduction in mean glucose was significantly larger for the eight participants with initial HbA1c >8.5% than for the 21 participants with initial HbA1c 6.5–7.5%. However, patients with an HbA1c in the target range at baseline were also able to considerably improve their glycaemic profiles with the sensor (Fig. 3). The reduction in hours per day >10.0 mmol/l was greater in participants with higher initial HbA1c values. A similar pattern could not be detected for hours per day <3.9 mmol/l, possibly because this measure of glycaemic control is more variable between participants.
Clinical benefits of 6 weeks of real-time CGM in a patient with type 1 diabetes and hypoglycaemia unawareness. Although HbA1c was within the target range at baseline (6.9%), the patient experienced significantly less time in the hypo- and hyperglycaemic range during real-time display of glucose values (c, d) than during a previous 3 week period with the device masked (a, b). The profile plots show the first week of the masked phase (a) and the last week of unmasked sensor wear (c). The glucose distribution histograms (b, d) include data for the whole phase. The patient improved his HbA1c to 6.4% during the study
In the hypoglycaemic fear survey, participants said that they tended to eat large snacks less frequently at bedtime after wearing the unmasked sensor than they had done at baseline and were more likely to keep their sugar high when they planned to be in a long meeting or at a party. Otherwise there were no other notable differences before and after wearing the sensor (Fig. 4).
Significant differences were observed only for two issues in the Hypoglycaemia Fear Survey before and during sensor wear, namely (a) for ‘Keep my sugar high when I plan to be in a long meeting or at a party’ (signed rank statistic of change in response for end of study–baseline 66.5, p = 0.021) and (b) for ‘Eat large snacks at bedtime’ (−66.0, p = 0.030). This indicates a tendency towards a safer feeling particularly at night, when wearing the sensor
Discussion
In contrast to the finding of the US trial [4], nearly all patients wearing the CGM system showed an improvement in several variables of HbA1c and glycaemic variability in the present study. A notable difference to the JDRF trial was that all but one patient in the present study wore the device for more than 85% of the time. Although this study does not allow conclusions to be drawn on what the patients changed to improve their control, continuous sensor use appears to be the single most critical point for success of CGM.
One particularly controversial finding in the JDRF trial [4] was the lack of an effect in the 15–24 and 8–14 years age groups. We therefore conducted an additional analysis of our data for this arbitrary cut-off point of above and below age 25 years (Table 3). Although our study was not powered to assess differences based on age, the differences in both groups point in the same direction and no significant differences were observed between the two groups in our study. As expected, the younger group had a higher HbA1c at onset, with a comparable decrease, than the patients aged 25 years and above. Of particular interest was the fact that glycaemic variability was comparable in both groups, but the reduction in glucose SD was less pronounced in the younger group. Thus, the age-related effects of glycaemic variability require further investigation, although they may just reflect age-related particularities of an unsteady lifestyle of young adults.
The importance of continuous sensor use is supported by a multi centre study using the Guardian RT system, with adult and paediatric participants who showed greater HbA1c improvement with continuous vs discontinuous use [21]. In contrast, the first paediatric and adult trials using a sensor-augmented pump system did not show an overall improvement of HbA1c [22, 23]. However, if the data were re-analysed in terms of sensor-use, patients wearing the sensor-augmented part of the system for most of the time did show a significant drop in their HbA1c. In the same way, a per-protocol analysis of the JDRF data failed to show an age-related effect of sensor-augmented therapy on HbA1c as the two younger age groups showed less sensor use than the older one [24]. Although no paediatric participants took part in the present trial, as the device was not labelled for use in this age group at the time of the study, it is likely that it will be of use for patients below age 18 in those wearing it most of the time [25, 26]. All in all, selecting the right patient for CGM may be more challenging in paediatric patients and young adults, but it can be accomplished successfully as the present study shows.
There is substantial variability in individual mean glucose concentrations for a given HbA1c [27]. In the present study the improvement of glycaemic variability was observed over a wide range of baseline HbA1c. Thus selection of patients for this technology should not be based on HbA1c. Surprisingly the time spent in hypoglycaemia was not improved during the study. Several factors may have contributed to this. First, the overall improvement of HbA1c and lowering of mean blood glucose as such necessarily increase the risk of hypoglycaemia, possibly offsetting a potential benefit of better recognition of impending hypoglycaemia. Second, our participants developed a great trust in the device and may therefore have been more willing to let their sugar levels be closer to the hypoglycaemic range. This could also be reflected by the finding that dependence on night-time snacks decreased, as expressed in the hypoglycaemia fear survey. Thus, the lowering of mean glucose without increasing the degree of symptomatic hypoglycaemia clearly demonstrates the benefit of sensor use.
In conclusion, the present European study has shown a considerable age-independent effect of the CGM system in improving HbA1c and glycaemic variability. The emerging evidence for glycaemic variability playing a role in such different areas as behavioural changes, rates of hypoglycaemia or the development of vascular complications underscores the considerable clinical benefit of this device when used on a regular basis.
Abbreviations
- CGM:
-
Continuous glucose monitoring
- GRADE:
-
Glycaemic risk assessment diabetes equation
- HBGI:
-
High blood glucose index
- LBGI:
-
Low blood glucose index
- MAGE:
-
Mean amplitude of glycaemic excursions
References
Golicki DT, Golicka D, Groele L, Pankowska E (2008) Continuous glucose monitoring system in children with type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetologia 51:233–240
Danne T, Lange K, Kordonouri O (2008) Real-time glucose sensors in children and adolescents with type-1 diabetes. Horm Res 70:193–202
Feldman B, Brazg R, Schwartz S, Weinstein R (2003) A continuous glucose sensor based on wired enzyme technology: results from a 3-day trial in patients with type 1 diabetes. Diabetes Technol Ther 5:769–779
Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW et al (2008) Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 359:1464–1476
Monnier L, Colette C (2008) Glycemic variability: should we and can we prevent it? Diabetes Care 31:S150–S154
Krinsley JS (2008) Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med 36:3008–3013
Service FJ, Molnar GD, Rosevear, Ackermann E, Gatweood LC, Taylor WF (1970) Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 19:644–655
Monnier L, Mas E, Ginet C et al (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295:1681–1687
McDonnell CM, Northam EA, Donath SM, Werther GA, Cameron FJ (2007) Hyperglycemia and externalizing behavior in children with type 1 diabetes. Diabetes Care 30:2211–2215
Frier BM (2008) How hypoglycaemia can affect the life of a person with diabetes. Diabetes Metab Res Rev 24:87–92
Reach G (2008) Continuous glucose monitoring and diabetes health outcomes: a critical appraisal. Diabetes Technol Ther 10:69–80
Danne T, Lange K, Kordonouri O (2007) New developments in the treatment of type 1 diabetes in children. Arch Dis Child 92:15–19
Fava S (2008) Role of postprandial hyperglycemia in cardiovascular disease. Expert Rev Cardiovasc Ther 6:859–872
Brownlee M, Hirsch IB (2006) Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA 295:1707–1708
Hirsch IB, Armstrong D, Bergenstal RM et al (2008) Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM). Diabetes Technol Ther 10:232–244
Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J (1987) Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 10:617–621
Kubiak T, Hermanns N, Schreckling HJ, Kulzer B, Haak T (2006) Evaluation of a self-management-based patient education programme for the treatment and prevention of hypoglycaemia-related problems in type 1 diabetes. Patient Educ Couns 60:228–234
Bode B, Silver M, Weiss R, Martin K (2008) Evaluation of a continuous glucose system for home-use conditions. Manag Care 17:40–45
Clarke WL, Breton M, Brayman K, McCall A (2005) Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther 7:849–862
Hill NR, Hindmarsh PC, Stevens RJ, Stratton IM, Levy JC, Matthews DR (2007) A method for assessing quality of control from glucose profiles. Diabet Med 24:753–758
Deiss D, Bolinder J, Riveline JP et al (2006) Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 29:2730–2732
Halvorson M, Carpenter S, Kaiserman K, Kaufman F (2007) A pilot trial in pediatrics with the sensor-augmented pump: combining real-time continuous glucose monitoring with the insulin pump. J Pediatr 150:103–105
Hirsch IB, Abelseth J, Bode BW et al (2008) Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther 10:377–383
The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group (2009) Continuous glucose monitoring and type 1 diabetes. N Engl J Med 360:190–192
Wilson DM, Beck RW, Tamborlane WV et al; DirecNet Study Group (2007) The accuracy of the FreeStyle Navigator continuous glucose monitoring system in children with type 1 diabetes. Diabetes Care 30:59–64
Wilson D, Chase HP, Kollman C, Diabetes Research in Children Network (DirecNet) Study Group et al (2008) Low-fat vs. high-fat bedtime snacks in children and adolescents with type 1 diabetes. Pediatr Diabetes 9:320–325
The Diabetes Research in Children Network (DirecNet) Study Group (2008) The relationship of A1c to glucose concentrations in children with type 1 diabetes: assessments by high frequency glucose determinations by sensors. Diabetes Care 31:381–385
Acknowledgements
This study received support from Abbott Diabetes Care, Oxford, UK. The authors wish to thank the diabetes teams and patients for their enthusiastic support of the study and M. de Wit, Diabetes Psychology Research Group, Department of Medical Psychology, VU University Medical Centre Noord, Amsterdam, the Netherlands for help with the Dutch Hypoglycaemia Fear Survey.
Duality of interest
Although protocol development (J. R. Bugler) and statistical analysis (Z. K. Welsh) were done in collaboration with staff from Abbott Diabetes Care, the complete data set and the codes for analysing the data were available to the study group. T. Danne drafted the manuscript. All authors participated in the data analysis and the writing of the manuscript. Therefore this study was conducted in accordance with the principles of the CONSORT statements and the authors declare that there is no further duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danne, T., de Valk, H.W., Kracht, T. et al. Reducing glycaemic variability in type 1 diabetes self-management with a continuous glucose monitoring system based on wired enzyme technology. Diabetologia 52, 1496–1503 (2009). https://doi.org/10.1007/s00125-009-1408-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1408-6