Abstract
Aims/hypothesis
Insulin resistance is caused by numerous factors including inflammation. It is characterised by defective insulin stimulation of adipocyte and muscle glucose transport, which requires the glucose transporter GLUT4 translocation towards the plasma membrane. Defects in insulin signalling can cause insulin resistance, but alterations in GLUT4 trafficking could also play a role. Our goal was to determine whether proteins controlling GLUT4 trafficking are altered in insulin resistance linked to obesity.
Methods
Using real-time RT-PCR, we searched for selected transcripts that were differentially expressed in adipose tissue and muscle in obese mice and humans. Using various adipocyte culture models and in vivo mice treatment, we searched for the involvement of TNF-α in these alterations in obesity.
Results
Sortilin mRNA and protein were downregulated in adipose tissue from obese db/db and ob/ob mice, and also in muscle. Importantly, sortilin mRNA was also decreased in morbidly obese human diabetic patients. Sortilin and TNF-α (also known as TNF) mRNA levels were inversely correlated in mice and human adipose tissues. TNF-α decreased sortilin mRNA and protein levels in cultured mouse and human adipocytes, an effect partly prevented by the peroxisome proliferator-activated receptor γ activator rosiglitazone. TNF-α also inhibited adipocyte and muscle sortilin mRNA when injected to mice.
Conclusions/interpretation
Sortilin, an essential player in adipocyte and muscle glucose metabolism through the control of GLUT4 localisation, is downregulated in obesity and TNF-α is likely to be involved in this defect. Chronic low-grade inflammation in obesity could thus contribute to insulin resistance by modulating proteins that control GLUT4 trafficking.
Similar content being viewed by others
Introduction
Insulin plays a crucial role in metabolic homeostasis by inhibiting hepatic glucose production and by increasing the disposal of postprandial glucose into adipocytes and muscles. Insulin induces translocation of the glucose transporter GLUT4 from intracellular organelles to the plasma membrane thus increasing glucose uptake [1, 2], a process which is altered in insulin resistance [3, 4]. The precise mechanisms underlying this defective insulin action are not fully understood, but certainly result from alterations in the pathway leading to the stimulation of glucose transport by insulin, i.e. alterations located between the insulin receptors and GLUT4.
Low-grade inflammation is associated with obesity and is tightly linked to insulin resistance [5]. Many inflammatory cytokines have been involved in the alteration of insulin signalling, thus providing molecular links between inflammation and insulin resistance [6]. Inflammation causes deregulation of the IRS, as reviewed [7, 8], with consequences in activation of the phosphatidylinositol 3-kinase/Akt pathway, which is essential for insulin-stimulated glucose transport [9]. However, partial inhibition of IRS function is not sufficient to have a major effect on insulin-induced glucose transport, since insulin action is normal in Irs1 +/− mice [10] or in mice with a partial knockdown of Irs1 in skeletal muscle [11]. In accordance, it has recently been established by studying various in vitro and in vivo insulin-resistant models that the most deleterious defects in insulin action could also occur independently of IRS proteins [3].
The decrease in insulin-induced glucose transport occurring in adipocytes from insulin-resistant patients could also result from a decrease in GLUT4 levels [12, 13]. In addition, alterations in GLUT4 localisation have been revealed by differences in sedimentation properties of GLUT4-containing structures in adipocytes and also in muscle from diabetic patients [14–16]. This GLUT4 mislocalisation could contribute to altered insulin-induced glucose transport because the ability of insulin to recruit GLUT4 at the plasma membrane results from the specific characteristics of GLUT4 trafficking [17]. Perturbations of this trafficking through the use of mutations in key motifs of the transporters result in altered insulin-induced GLUT4 recruitment from intracellular compartments to the plasma membrane [18]. However, the mechanisms involved in the perturbation of GLUT4 localisation in insulin resistance are currently unknown, but we made the hypothesis that alterations in the levels of proteins involved in the control of GLUT4 trafficking could play a role.
Considerable progress has been made on the identification of proteins involved in the control of GLUT4 trafficking [17]. For example, it is now well-established that the vesicle/target soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex (vesicle-associated membrane protein [VAMP]2/syntaxin 4) and its regulators (mammalian homologue of Caenorhabditis elegans unc-18 isoform c [MUNC18C], syntaxin 4-binding protein [SYNIP]) play an essential role in the insulin-regulated tethering/fusion of GLUT4-containing vesicles from the insulin-responsive compartment (IRC) with the plasma membrane [19]. Several proteins, such as Golgi-localising γ-adaptin ear homology domain, ARF-binding protein (GGA)-1, sortilin and syntaxin 6 have also been identified to play a role in GLUT4 targeting to the IRC and possibly also in the genesis of this specific compartment in cultured adipocytes [20–22]. Interestingly, in vivo deregulation of the expression of Munc18c or Stx4 causes decreased whole-body insulin sensitivity and also muscle insulin resistance [23, 24]. It remains to be determined whether such abnormal regulations exist in insulin-resistant situations.
In the present study, we examined whether levels of key proteins involved in the control of GLUT4 trafficking are altered in obesity. We found that levels of sortilin are decreased in adipose tissues of morbidly obese humans and mice, and in skeletal muscle of obese mice. We provide evidence for a role of TNF-α in the downregulation of the gene encoding sortilin (Sort1) expression and sortilin production. Our data thus suggest that the inflammatory state linked to obesity could contribute to insulin resistance by modulating levels of proteins involved in GLUT4 trafficking.
Methods
Antibodies
Antibodies against sortilin were from BD Biosciences (San Jose, CA, USA) whereas antibodies against tubulin were from Sigma-Aldrich (St Louis, MO, USA). Horseradish peroxidase-coupled anti-species antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
Primers for real-time PCR
Primers were designed using Primer Express software (Applied Biosystems, Austin, TX, USA) and synthetised by Eurogentec (Seraing, Belgium). A list is available in Electronic supplementary material (ESM) Table 1. Each primer couple was validated by characterising the slopes of C t over a range of three log dilutions and the curve of temperature dissociation of the amplicons.
Reagents
TNF-α and IL6 were obtained from PeProtech (Neuilly sur Seine, France). Rosiglitazone was from Axxora, LLC (San Diego, CA, USA). Trizol and cultured medium were from Invitrogen SARL (Cergy Pontoise, France). Other chemicals were from Sigma-Aldrich.
Human subcutaneous adipose tissues biopsies
Morbidly obese women were selected through the Department of Digestive Surgery, where they underwent elective bariatric surgery. We selected for this study six obese women (age 32.0 ± 8.5 years; BMI 44.25 ± 7.16 kg/m2, p < 0.01 vs control; fasted glycaemia 4.99 ± 0.49 mmol/l) and six obese diabetic women (age 49.0 ± 7.1 year; BMI 48.34 ± 7.16 kg/m2, p < 0.01 vs control; fasted glycaemia: 9.28 ± 3.65 ↑ mmol/l, p < 0.01 vs control and vs obese). Biopsies were performed on four control women subjected to abdominal surgery or lipectomy for cosmetic purpose (age 37.2 ± 11.3 year; BMI 20.89 ± 0.50 kg/m2; fasting plasma glucose 5.08 ± 1.44 mmol/l). None of the obese diabetic patients were being treated with thiazolidinediones. The study was performed according to French legislation regarding ethics and human research (Huriet–Serusclat law, DGS 2003/0395). Informed consent was obtained from all participants. During surgery, surgical subcutaneous adipose tissue biopsies were obtained and immediately frozen in liquid nitrogen and stored at −80°C.
Human adipose tissue explants
Adipose tissue explants were obtained following aesthetic abdominoplasty from former morbidly obese patients 2 years after a gastric bypass when their body weight had stabilised and their systemic inflammation was widely improved. Small pieces of adipose tissue were dissected and incubated for 48 h in presence of the indicated drugs in DMEM medium containing 2% BSA (wt/vol.) and 100 µmol/l gentamicin.
Animals
Male ob/ob and db/db mice and their lean control littermates (ob/+ and db/+) were purchased from Charles River Laboratories (St Aubin les Elbeuf, France) and housed at the animal facility of the Faculty of Medicine (Nice, France). Mice were maintained on a 12 h light–dark cycle and provided free access to water and standard rodent show. At 13 weeks of age mice were killed by cervical dislocation and epididymal fat pads and skeletal muscle were removed and freeze-clamped in liquid nitrogen. We used five db/+ mice (weight 28.3 ± 0.8 g; fed glycaemia 10.0 ± 0.3 mmol/l), five db/db mice (weight 48.5 ± 1.8 g, fed glycaemia 31.6 ± 0.5 mmol/l, p < 0.001 for difference between obese mice and lean littermates), ten ob/+ mice (weight 30.4 ± 0.7 g; fed glycaemia 9.3 ± 0.3 mmol/l) and 15 ob/ob mice (weight: 46.9 ± 1.1 ↑ g, fed glycaemia 17.5 ± 1.1 mmol/l, p < 0.001 for difference between obese mice and lean littermates). Male C57BL/6J mice were deprived of food in the morning and injected intraperitoneally with TNF-α (4 µg) 8 h later. Saline solution was used as control. Mice were killed 16 h after injection and epididymal adipose tissues and skeletal muscles were removed. Principles of laboratory animal care were followed and the Ethical Committee of the Faculty of Medicine approved the animal experiments.
Cells
3T3-L1 fibroblasts were cultured in DMEM–10% (vol./vol.) FCS and induced to differentiate in adipocytes as previously described [25]. Briefly, 3 days after confluence, fibroblasts were treated for 2 days with DMEM–10% FCS (vol./vol.) supplemented with isobutyl methylxanthine (250 nmol/l), dexamethasone (250 nmol/l) and insulin (800 nmol/l), and then for two additional days with DMEM–10% FCS containing 800 nmol/l insulin. Cells were used between day 2 and 7 after the end of the differentiation protocol when the adipocyte phenotype appeared in more than 90% of the cells.
Human pre-adipocytes (Biopredic, Rennes, France) were grown as previously described [12]. Differentiation was induced after confluence by changing the medium for DMEM–Ham’s F12–HEPES (15 mmol/l), containing 2 mmol/l l-glutamine, 3% FCS (vol./vol.), 33 µmol/l biotin, 100 nmol/l insulin, 17 µmol/l pantothenate, 200 nmol/l isobutylmethylxanthine, 1 µmol/l dexamethasone and 10 µmol/l rosiglitazone. The medium was removed after 3 days and replaced with Ham’s F12–HEPES (15 mmol/l), 2 mmol/l l-glutamine and 10% FCS (vol./vol.), supplemented with biotin (33 µmol/l), insulin (100 nmol/l), pantothenate (17 µmol/l) and dexamethasone (1 µmol/l). Then the cells were fed every 2 days with the same medium. Human adipocytes were used 10 to 15 days after the end of the differentiation protocol. More than 90% of the cells presented an adipocyte phenotype.
Real-time PCR
RNAs were prepared using a kit (RNeasy total RNA; Qiagen, Courteboeuf, France), treated with DNAse (Applied Biosystems) and used to synthetise cDNAs with a kit (cDNA High-capacity Archive Kit; Applied Biosystems). Real-time quantitative PCR was performed with sequence detection systems (ABI PRISM 7000 or 7500; Applied Biosystems) and SYBR green dye as described previously [12]. mRNA levels were expressed relative to RPLP0 or Rplp0, which was used as an invariant gene (ΔC t = C tR − C tRPLP0). The relative amount of mRNA between two groups was determined by using the second derivative maximum method. The results were expressed relative to the mean of the group of controls which was arbitrarily assigned to a value of 1.
Western blots
Total homogenates were prepared in Laemmli buffer from adipocytes, adipose tissues or skeletal muscles. Proteins (40 µg) were separated by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes and incubated with anti-sortilin antibodies. Horseradish peroxidase-coupled anti-species antibodies were then added and chemiluminescence was detected using Fujifilm Las-3000 apparatus (Fujifilm Life Science, F.S.V.T, Courbevoie, France). The same membrane was re-probed with anti-tubulin antibodies as a loading control. Signal quantification was performed using the MultiGauge software (Fujifilm Life Science) and sortilin level was normalised to that of tubulin.
Statistical analysis
Statistical significance between two groups (mice or human) was determined using non-parametric Kruskal–Wallis test. p < 0.05 was considered to be significant. For the analysis of the significance between various cell treatments, we used Student’s paired t test. Search for correlation between two variables was performed by Pearson analysis on ranks. The analyses were performed with MINITAB software (Minitab, State College, PA, USA).
Results
The abundance of sortilin is decreased in white adipose tissue and skeletal muscle of obese db/db mice compared with their lean db/+ littermates
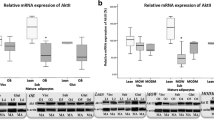
We first aimed to determine whether the production of key proteins involved in the control of GLUT4 intracellular trafficking was modified in the db/db mice, a genetic model of murine obesity and diabetes. We focused on: (1) the vesicle SNARE/target SNARE complex and their regulators, i.e. VAMP2, syntaxin 4, MUNC18C, SYNIP, which play an essential role in the insulin-regulated tethering/fusion of GLUT4-containing vesicles from the IRC to the plasma membrane; (2) proteins involved in GLUT4 targeting to the IRC, i.e. GGA1, sortilin, syntaxin 6; and (3) the protein AS160 involved at the crossroad of insulin signalling and GLUT4 trafficking, as reviewed [2, 26]. We observed no significant differences between lean and obese mice in the mRNA levels for all proteins studied except for Sort1, which encodes sortilin (Table 1). As recently described in human obesity [27], we found that Munc18c expression was not altered in muscle from db/db mice. Sort1 expression was decreased by ~25 and 35% in white adipose tissue and skeletal muscle, respectively (Table 1, Fig. 1a). As expected, we observed a profound decrease in the expression of Irs1 mRNA in obese animals compared with their lean controls [28]. Sort1 mRNA expression was also decreased in obese ob/ob mice (0.72 ± 0.09) compared with ob/+ lean mice (1.00 ± 0.04). The amount of sortilin protein was decreased by nearly 70% in white adipose tissue and skeletal muscle of obese db/db mice compared with lean db/+ mice (Fig. 1b, c). Taken together, our results suggest that the decrease in sortilin levels in db/db mice could result from defects at the mRNA and protein levels.
The expression of sortilin is downregulated in adipose tissues and skeletal muscles of obese and diabetic db/db mice. a Total RNAs were prepared from epidydimal white adipose tissues (WAT) and hindleg skeletal muscles (SkM) of six db/+ (white columns) and six db/db mice (back columns). Expression of Sort1 mRNA was analysed by real-time quantitative PCR, normalised to Rplp0 mRNA and expressed in arbitrary units with control values taken as 1. *p < 0.05. b, c Homogenates were prepared from the same tissue samples of the mice. Proteins (50 µg) were separated by SDS-PAGE, transferred to a PVDF membrane and incubated with anti-sortilin mAb antibodies followed by horseradish peroxidase-coupled anti species antibodies. Detection was by chemiluminescent reagent and a Fujifilm Las-3000 device. Quantification of sortilin amount (b) was normalised to tubulin expression from six db/+ and six db/db mice. c Representative immunodetection of sortilin and tubulin as loading control for three different mice analysed on the same gel. ***p < 0.001. d Tnf-α and Sort1 mRNA levels were quantified in control db/+ (white triangles) and ob/+ (white circles) lean mice, and obese db/db (black triangles) and ob/ob (black circles) mice. A correlation analysis between expression levels of these two mRNAs was performed as described in the Methods section. r = −0.661, p < 0.0001
Obesity is linked to low-grade inflammation that emanates to a large extent from the adipose tissue. Many of these inflammatory factors, including TNF-α, have documented inhibitory effects on insulin action in peripheral tissues, including the adipose tissue itself [6]. We thus determined whether the levels of Sort1 and Tnf-α mRNA are correlated in epidydimal adipose tissues of lean and obese mice and found a negative correlation (Fig. 1d) between the mRNA encoding for these two proteins. This inverse correlation between Sort1 and Tnf-α expression suggests that inflammation may be involved in the downregulation of sortilin in murine obesity.
TNF-α induces the downregulation of Sort1 mRNA expression and the decrease in sortilin production in adipocytes
Because Tnf-α and Sort1 mRNA expression were negatively correlated in mice adipose tissues, we investigated whether inflammatory cytokines (TNF-α and IL6) could regulate the expression of sortilin in cultured adipocytes, i.e. murine 3T3-L1 adipocytes and human adipocytes differentiated in culture from primary pre-adipocytes. For that, fully differentiated 3T3-L1 adipocytes were treated with TNF-α and the mRNA (Fig. 2a) and protein levels (Fig. 2b, c) of sortilin were determined by real-time PCR and western blotting, respectively. TNF-α induced a dose-dependent inhibition of Sort1 mRNA expression with a 75% inhibition at a concentration of 50 ng/ml. TNF-α (20 ng/ml) also induced a time-dependent decrease in sortilin protein levels (Fig. 2b, c). The inhibition reached 70% and 80% after 48 and 96 h of treatment respectively with TNF-α. Because TNF-α induces IL6 secretion in adipocytes [29], some of the TNF-α effects could have been indirectly mediated through this cytokine. However, this is unlikely, since IL6 did not alter Sort1 mRNA or sortilin levels (Fig. 2a, c), while it did inhibit mRNA expression of Glut4 (also known as Slc2a4) (Fig. 2a) and largely increased as expected [29] that of Glut1 (also known as Slc2a1) (data not shown).
TNF-α inhibits Sort1 mRNA and decreases sortilin levels in 3T3-L1 adipocytes. a 3T3-L1 adipocytes were treated for 48 h without (NT) or with increasing concentrations of TNF-α (2, 5, 10, 20 and 50 ng/ml) or with 50 ng/ml of IL6. The expression levels of Sort1 mRNA (black columns) were analysed by real-time quantitative PCR, normalised to Rplp0 mRNA and expressed in arbitrary units with control values taken as 1. *p < 0.05. Glut4 mRNA (white columns) was also quantified upon IL6 treatment as a positive control for IL6 action. b 3T3-L1 adipocytes were treated with nothing (NT) or TNF-α 20 ng/ml) for the indicated periods of time. Homogenates were prepared and 40 µg of proteins were separated by SDS-PAGE, transferred to PVDF membrane and probed with anti-sortilin mAb and anti-tubulin mAb as a loading control. Western blots (WB) from a representative experiment are shown. c 3T3-L1 adipocytes were treated as above (b) with 20 ng/ml TNF-α (squares) or with 50 ng/ml of IL6 (circles) during the same periods of time. Values are mean ± SEM of the quantification of sortilin normalised to that of tubulin from three independent experiments. *p < 0.05
TNF-α (20 ng/ml) was similarly effective in inducing inhibition of SORT1 mRNA expression (Fig. 3a) and sortilin protein levels (Fig. 3b) in human adipocytes. TNF-α is thus able to decrease the amount of sortilin in murine and human adipocytes mainly through the regulation of its mRNA levels.
TNF-α inhibits SORT1 mRNA and protein levels in human adipocytes differentiated in culture. a Cultured human adipocytes were treated for 48 h with 20 ng/ml of TNF-α. SORT1 mRNA levels were determined by real-time PCR, normalised to the levels of RPLP0 mRNA and expressed in arbitrary units as the mean ± SEM of three independent experiments, *p < 0.05. b Cultured human adipocytes were treated with 20 ng/ml of TNF-α for increasing periods of time. Homogenates were prepared and the amount of sortilin and tubulin were determined by western blotting (WB). A representative experiment is shown
Rosiglitazone partially prevents TNF-α-induced inhibition of sortilin production in 3T3-L1 adipocytes and human white adipose tissue explants
The deleterious effects of TNF-α on mature adipocytes are thought to be mediated largely through the inhibition of the nuclear hormone receptor peroxisome proliferator-activated receptor γ(PPARγ), an essential transcriptional regulator of adipogenesis also required for maintenance of mature adipocyte functions, as reviewed [30, 31]. We thus determined whether rosiglitazone, a thiazolidinedione with agonistic effects on PPARγ [32], could interfere with the effect of TNF-α on sortilin levels. 3T3-L1 adipocytes were treated for 48 h with TNF-α and without or with 1 or 10 µmol/l of rosiglitazone (Fig. 4a–c). As described above, TNF-α induced a decrease in Sort1 mRNA and protein levels. Rosiglitazone (1 µmol/l) partially prevented the effect of TNF-α at both the mRNA (Fig. 4a) and protein levels (Fig. 4b, c), although it also slightly inhibited sortilin production itself. The extent of prevention was identical when a higher concentration of rosiglitazone was used, thus indicating that its protective action against the inhibitory effect of TNF-α is only partial.
Rosiglitazone partially prevents the TNF-α-induced inhibition of sortilin expression. a–c 3T3-L1 adipocytes were treated without or with the indicated concentration of rosiglitazone (Rosi) in presence or not of TNF-α for 48 h. Total RNA or homogenates were prepared and the amount of Sort1 mRNA (a) or sortilin protein (b, c) were determined as described previously. b Mean ± SEM of three independent experiments, (c) representative western blots (WB). d Human white adipose tissue explants were treated with nothing or rosiglitazone in presence or not of TNF-α. After 48 h total RNA was prepared and the levels of SORT1 mRNA determined by real-time PCR. Values are mean ± SEM of the results obtained with subcutaneous adipose tissue from six individuals, each condition being performed in duplicate. *p < 0.05 for difference from TNF-α condition; †p < 0.05 for difference from condition without rosiglitazone
A 48 h treatment of human adipose tissue explants with 10 ng/ml of TNF-α also induced a 60% decrease in the expression of SORT1 mRNA, as in cultured human and mouse adipocytes; this decrease was partially prevented by the addition of 1 ↑ µmol/l rosiglitazone (Fig. 4d). The protective effect of rosiglitazone against the deleterious effect of TNF-α thus occurs not only in adipocytes but also in adipose tissue. Taken together, our results suggest that the action of TNF-α on sortilin expression is at least in part prevented by PPARγ activation.
TNF-α-treated mice express lower levels of Sort1 mRNA in white adipose tissue and skeletal muscle
Our previous results showed that in in vitro systems TNF-α induced the inhibition of sortilin expression. It is known that the expression of TNF-α mRNA is increased in adipose tissues from obese animals and patients [33, 34] (Fig. 2), as are TNF-α plasma levels in obese animal models [35]. We thus aimed to determine whether TNF-α could induce a decrease in expression of Sort1 transcripts in vivo. To this aim, mice were treated with TNF-α or vehicle and killed 16 h after injection. Epididymal adipose tissue and hindleg skeletal muscles were removed, total RNA was prepared and sortilin mRNA levels determined by real-time PCR. As shown in Fig. 5a, Sort1 mRNA expression was decreased by 40% in adipose tissue and by nearly 20% in skeletal muscle following TNF-α treatment. In accordance with the involvement of PPARγ in the inhibitory effect of TNF-α on sortilin expression, we found a positive correlation between Pparg and Sort1 expression in the adipose tissues of TNF-α-treated mice (Fig. 5b).
Sortilin mRNA expression in white adipose tissues and skeletal muscle is inhibited in vivo by the injection of TNF-α. a Total RNA was prepared from epidydimal white adipose tissues and skeletal muscle of mice injected with vehicle (white columns) or TNF-α (black columns) as indicated in the Methods section. mRNA levels of sortilin were determined by real-time PCR, normalised to the levels of Rplp0 mRNA and expressed in arbitrary units as the mean ± SEM of 14 mice in the control group and of 16 mice in the TNF-α-treated group. *p < 0.05. b The expression of Pparg was determined in white adipose tissues of control and TNFα-treated mice, normalised to the level of Rplp0 mRNA and expressed relative to the mean of control animals as for sortilin mRNA expression. We then performed a correlation analysis between the levels of expression of Pparg and Sort1 as indicated in the Methods section. r = 0.629, p < 0.01
The expression of sortilin mRNA is decreased in subcutaneous adipose tissue of morbidly obese diabetic patients compared with lean controls
Our results suggest that the decreased levels of sortilin in obese animals involved TNF-α. Because we found that TNF-α was also able to decrease sortilin expression in human adipocyte and adipose tissue, we next determined whether SORT1 mRNA expression was modified in subcutaneous adipose tissue from obese patients (Fig. 6a). We observed a 40% decrease in the amount of SORT1 mRNA in subcutaneous adipose tissues from morbidly obese diabetic patients compared with lean individuals. There was also a trend towards a decrease in the group of obese, non-diabetic patients compared with control. However, there was no correlation between SORT1 mRNA levels and fasted glycaemia (data not shown), suggesting that hyperglycaemia as such is not the cause of this difference. Interestingly, we found a significant negative correlation between the expression of SORT1 and TNF-α transcripts in human adipose tissue, as in mice (Fig. 6), strongly suggesting that inflammation could also be responsible for sortilin level changes in human obesity.
The expression of SORT1 mRNA is downregulated in adipose tissues of obese diabetic patients and is inversely correlated with the expression of TNF-α mRNA. a Total RNA was prepared from subcutaneous adipose tissue of control lean participants (white columns) and morbidly obese patients with (black columns) or without (hatched columns) diabetes. The mRNA levels of SORT1 were determined by real-time PCR, normalised to the levels of RPLP0 mRNA and expressed in arbitrary units. *p < 0.05. b The mRNA levels of TNF-α were determined as for SORT1 mRNA in the same total RNA samples and a correlation analysis was performed, as described in the Methods section, between SORT1 and TNF-α expression in lean (white circles), obese (white triangles) and obese diabetic (black circles) patients. r = −0.559, p < 0.02
Discussion
In the present paper, we found that sortilin expression was downregulated in adipose tissue and muscles from genetically obese mice and morbidly obese humans. Sortilin is a major component of the IRC in adipocytes [36] and plays an essential role in the development of the insulin-responsive transport system in cultured adipocytes and muscle cells [21, 37]. Sortilin belongs to a class of type I receptors characterised by the presence of a luminal/extracellular region containing a cysteine-rich domain homologous to the yeast vacuolar sorting 10 protein and cytoplasmic sequences involved in binding to GGA adaptors [38, 39]. Through the use of these domains, it acts as a Golgi-endosome sorting receptor for some lysosomal proteins [40] and as a Golgi-IRC sorting receptor for GLUT4 and insulin-regulated aminopeptidase (IRAP) [21, 41, 42]. Thus, an attractive hypothesis would be that the decreased amounts of sortilin observed by us in morbidly obese mice and individuals could contribute to defects in glucose transport in adipocyte and muscle. Interestingly, in cultured adipocytes, decreasing sortilin amounts through the use of specific short-hairpin RNA induced inhibition of insulin-induced glucose transport and of the biogenesis of the IRC in an in vitro reconstitution assay [21]. In C2C12 myocytes, the development of insulin-induced glucose transport was enhanced by the overproduction of sortilin [37]. Furthermore, this sortilin-dependent perturbation of GLUT4 trafficking could alter GLUT4 stability, thus amplifying glucose uptake defects. Indeed, it has been reported that decreasing the amount of sortilin leads to a decrease in the amount of GLUT4 [21].
The decrease in sortilin levels in obesity results from defects in the regulation of its mRNA and protein. Indeed, we observed an 80% decrease in the amount of the protein, while the mRNA was only decreased by 30 to 40%. We identified TNF-α as an agent that controls Sort1 mRNA expression. We found that it inhibited the expression of Sort1 mRNA in vitro in cultured murine and human adipocytes, and in vivo after injection into the mice. We also found that IL1β, which had increased production in obesity and which induced adipocyte insulin resistance [12], also inhibited Sort1 mRNA expression in 3T3-L1 adipocytes (data not shown), thus suggesting that obesity-linked inflammation could be involved in the downregulation of SORT1 mRNA expression. By contrast, TNF-α is probably not involved in the mechanisms that would explain the more important downregulation of the protein in obese db/db mice. In 3T3-L1 adipocytes, we observed only a slightly more effective downregulation of the protein than of mRNA expression by TNF-α (60% inhibition of mRNA, 70% inhibition of the protein after 48 h with 20 ng/ml TNF-α). To the best of our knowledge, our study demonstrates for the first time that inflammatory stimuli could target proteins involved in GLUT4 trafficking.
We found that treatment with rosiglitazone, an activator of PPARγ, slightly inhibited expression of sortilin, but more importantly partially prevented the TNF-α-induced decrease in Sort1 expression in mouse and human adipocytes. This prevention is only partial, either because TNF-α uses a PPARγ and a PPARγ-independent mechanism to inhibit expression of sortilin or because rosiglitazone only partly prevents the deleterious effects of TNF-α on PPARγ. Regarding the second hypothesis, TNF-α has been shown to regulate PPARγ both at the level of its production and of its activation [31]. In mature adipocytes, thiazolidinedione did not prevent TNF-α-induced decrease in PPARγ amount [43], but would be able to rescue the inhibition of PPARγ activity. However, we cannot exclude that this protective effect of rosiglitazone could be independent of PPARγ, as already described for some effects of thiazolidinediones [44]. Whatever the explanations, it should now be considered that the insulin-sensitising effect of thiazolinediones on mature insulin-resistant adipocytes could involve the upregulation of sortilin, which would enhance insulin-induced glucose transport.
The decreased expression of sortilin could also contribute to insulin resistance because sortilin plays other roles than controlling GLUT4 trafficking. Sortilin binds the lipoprotein lipase at the plasma membrane and targets it towards the degradation pathway [45]. A decrease in sortilin amount could augment fatty acid availability in muscle, thus increasing lipotoxic effects, and could favour adipocyte hypertrophy. Sortilin is a co-receptor for the p75 neurotrophin receptor (p75NTR), allowing high-efficiency pro nerve growth factor [NGF] binding [46]. This pathway is involved in terminal myocyte differentiation and is consequently involved in development of the insulin-responsive glucose transport system in muscle [37]. Such a role in adipocytes remains to be explored. However, NGF and the receptor p75NTR/sortilin are produced by adipose tissue [36, 47], and both circulating NGF [48] and sortilin levels (present study) are modified in obesity. Sortilin is also the neurotensin-3 receptor, but the physiological role of this neurotensin-activated pathway is not fully understood [38]. The potential involvement of these sortilin-dependent pathways in the complications of obesity require further investigations.
In summary, our results demonstrate that sortilin, a protein involved in GLUT4 trafficking and possibly in other functions important for muscle and adipocyte biology, is downregulated in obesity in an inflammation-dependent manner. These data therefore identify a new molecular mechanism that could link the chronic low-grade inflammatory state and the development of insulin resistance in obesity.
Abbreviations
- GGA:
-
Golgi-localising γ-adaptin ear homology domain, ARF-binding protein
- IRC:
-
Insulin-responsive compartment
- MUNC18:
-
Mammalian homologue of Caenorhabditis elegans unc-18
- NGF:
-
Nerve growth factor
- p75NTR:
-
p75 Neurotrophin receptor
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- PVDF:
-
Polyvinylidene difluoride
- SYNIP:
-
Syntaxin 4-interacting protein
- SNARE:
-
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- VAMP:
-
Vesicle-associated membrane protein
References
Bryant NJ, Govers R, James DE (2002) Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol 3:267–277
Kaddai V, Le Marchand-Brustel Y, Cormont M (2008) Rab proteins in endocytosis and Glut4 trafficking. Acta Physiol 192:75–88
Hoehn KL, Hohnen-Behrens C, Cederberg A et al (2008) IRS1-independent defects define major nodes of insulin resistance. Cell Metab 7:421–233
Mueckler M (1993) The molecular biology of glucose transport: relevance to insulin resistance and non-insulin dependent diabetes mellitus. J Diabetes Complications 7:130–141
Clement K, Langin D (2007) Regulation of inflammation-related genes in human adipose tissue. J Intern Med 262:422–430
Anderson CX, Gustafson B, Hammerstedt A, Hedjazifar S, Smith U (2008) Inflamed adipose tissue, insulin resistance and vascular injury. Diabetes Metab Res Rev 24:595–603
Gual P, Le Marchand-Brustel Y, Tanti JF (2005) Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87:99–109
Zick Y (2005) Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE: pe4
Ishiki M, Klip A (2005) Recent developments in the regulation of glucose transporter-4 traffic: new signals, locations and partners. Endocrinology 146:5071–5078
Shirakami A, Toyonaga T, Tsuruzoe K et al (2002) Heterozygous knockout of the IRS-1 gene in mice enhances obesity-linked insulin resistance: a possible model for the development of type 2 diabetes. J Endocrinol 174:309–319
Cleasby ME, Reinten TA, Cooney GJ, James DE, Kraegen EW (2007) Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression. Mol Endocrinol 21:215–228
Jager J, Grémeaux T, Cormont M, Le Marchand-Brustel Y, Tanti J-F (2007) Interleukin-1β-induced insulin resistance in adipocytes through downregulation of IRS-1 expression. Endocrinology 148:241–251
Hotamisligil GS (1999) The role of TNFα and TNF receptors in obesity and insulin resistance. J Intern Med 245:621–625
Garvey WT, Maianu L, Zhu J-H, Hancock JA, Golichowski AM (1993) Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes. Heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters. Diabetes 42:1773–1785
Garvey WT, Maianu L, Zhu J-H, Brechtel-Hook G, Wallace P, Baron AD (1998) Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest 101:2377–2386
Maianu L, Keller SR, Garvey WT (2001) Adipocytes exhibit abnormal subcellular distribution and translocation of vesicles containing glucose transporter 4 and insulin-regulated aminopeptidase in type 2 diabetes mellitus: implication regarding defects in vesicle trafficking. J Clin Endocrinol Metab 86:5450–5456
Larance M, Ramm G, James DE (2008) The GLUT4 code. Mol Endocrinol 22:226–233
Blot V, McGraw TE (2008) Molecular mechanisms controlling GLUT4 intracellular retention. Mol Biol Cell 19:3477–3487
James DE (2005) MUNC-ing around with insulin action. J Clin Invest 115:219–221
Watson RT, Kahn A, Furukawa M et al (2004) Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent. EMBO J 19:2059–2070
Shi J, Kandror KV (2005) Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev Cell 9:99–108
Perera HK, Clarke M, Morris NJ, Hong W, Chamberlain LH, Gould GW (2003) Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol Biol Cell 14:2946–2958
Yang C, Coker KJ, Kim JK et al (2001) Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J Clin Invest 107:1311–1318
Oh E, Spurlin BA, Pessin JE, Thurmond DC (2005) Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes 54:638–647
Kaddai V, Gonzalez T, Bolla M, Le Marchand-Brustel Y, Cormont M (2008) The nitric oxide-donating derivative of acetylsalicylic acid, NCX 4016, stimulates glucose transport and glucose transporters translocation in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 192:E162–E169
Sakamoto K, Holman GD (2008) Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab 295:E29–E37
Bergman BC, Cornier M-C, Horton TJ, Bessenen DH, Eckel RH (2008) Skeletal muscle munc18c and syntaxin 4 in human obesity. Nutr Metab (Lond) 5:21–27
Lee YH, White MF (2004) Insulin receptor substrate proteins and diabetes. Arch Pharm Res 27:361–370
Rotter V, Nagaev I, Smith U (2003) Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-α, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 278:45777–45784
Guilherme A, Virbasius JV, Puri V, Czech MP (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9:367–377
Ye J (2008) Regulation of PPARγ function by TNF-α. Biochem Biophys Res Commun 374:405–408
Forman BM, Chen J, Evans RM (1996) The peroxisome proliferator-activated receptors: ligands and activators. Ann N Y Acad Sci 804:266–275
Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95:2409–2415
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259:87–91
Sethi JK, Hotamisligil GS (1999) The role of TNF alpha in adipocyte metabolism. Semin Cell Dev Biol 10:19–29
Lin BZ, Pilch PF, Kandror KV (1997) Sortilin is a major protein component of Glut4-containing vesicles. J Biol Chem 272:24145–24147
Ariga M, Nedachi T, Katagiri H, Kanzaki M (2008) Functional role of sortilin in myogenesis and development of insulin-responsive glucose transport system in C2C12 myocytes. J Biol Chem 283:10208–10220
Mazella J, Vincent JP (2006) Functional roles of the NTS2 and NTS3 receptors. Peptides 27:2469–2475
Tooze SA (2001) Cell Biology. GGAs tie up the loose ends. Science 292:1663–1665
Ni X, Canuel M, Morales CR (2006) The sorting and trafficking of lysosomal proteins. Histol Histopathol 21:899–913
Shi J, Kandror KV (2007) The luminal domain Vps10 domain of sortilin plays the predominant role in targeting the insulin-responsive Glut4-containing vesicles. J Biol Chem 282:9008–9016
Li LV, Kandror KV (2005) Golgi-localized, gamma-ear-containing, Arf-binding protein adaptors mediate insulin-response trafficking of glucose transporter 4 in 3T3-L1 adipocytes. Mol Endocrinol 19:2145–2153
Rosenbaun SE, Greenberg AS (1998) The short- and long-term effects of tumor necrosis factor-alpha and BRL 49653 on peroxisome proliferator-activated receptor (PPAR)gamma2 gene expression and other adipocyte genes. Mol Endocrinol 12:1150–1160
Fürnsinn C, Waldhäusl W (2002) Thiazolidinediones: metabolic actions in vitro. Diabetologia 45:1211–1223
Nielsen MS, Jacobsen C, Olivecrona G, Gliemann J, Petersen CM (1999) Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem 274:8832–8836
Bronfman FC, Fainzilber M (2004) Multi-tasking by the p75 neurotrophin receptor: sortilin things out? Embo Rep 5:867–871
Peeraully MR, Jenkins JR, Trayhurn P (2004) NGF gene expression and secretion in white adipose tissue: regulation in 3T3-L1 adipocytes by hormones and inflammatory cytokines. Am J Physiol Endocrinol Metab 287:E331–E339
Bullo M, Peeraully MR, Trayhurn P, Folch J, Salas-Salvado J (2007) Circulating nerve growth factor levels in relation to obesity and the metabolic syndrome in women. Eur J Endocrinol 157:303–310
Acknowledgements
This work was supported by the Institut National de la Santé et de la Recherche Médicale (Paris, France), the University of Nice-Sophia Antipolis (Nice, France) and the Programme Hospitalier de Recherche Clinique (Nice, France). This study was also supported by a grant from Takeda–ALFEDIAM (Paris, France) to M. Cormont. P. Gual received support from the French Research Ministry (ANR-05-PCOD-025602 and PNRHGE, Paris, France) and from charities (ALFEDIAM and AFEF/Schering-Plough, Paris, France). This work is part of the project Hepatic and Adipose Tissue and Functions in the Metabolic Syndrome (HEPADIP, see http://www.hepadip.org/), which is supported by the European Commission (Brussels, Belgium) as an Integrated Project under the 6th Framework Programme (Contract LSHM-CT-2005–018734). Y. Le Marchand-Brustel and P. Gual are recipients of an Interface grant with the Nice University Hospital (Nice, France). V. Kaddai was successively supported by the Fondation pour la Recherche Médicale (Paris, France) and by the Association pour la Recherche Contre le Cancer (Paris, France). S. Bonnafous was supported by ANR-05-PCOD-025-02 (French Ministry, Paris, France). We are grateful to M. Mari (Department of Cell Biology, Utrecht, Netherlands) for helpful discussions on sortilin functions.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
List of the primers used for real-time PCR (PDF 90.0 KB)
Rights and permissions
About this article
Cite this article
Kaddai, V., Jager, J., Gonzalez, T. et al. Involvement of TNF-α in abnormal adipocyte and muscle sortilin expression in obese mice and humans. Diabetologia 52, 932–940 (2009). https://doi.org/10.1007/s00125-009-1273-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-009-1273-3