Abstract
Aims/hypothesis
We determined whether fixed doses of benfotiamine in combination with slow-release α-lipoic acid normalise markers of reactive oxygen species-induced pathways of complications in humans.
Methods
Male participants with and without type 1 diabetes were studied in the General Clinical Research Centre of the Albert Einstein College of Medicine. Glycaemic status was assessed by measuring baseline values of three different indicators of hyperglycaemia. Intracellular AGE formation, hexosamine pathway activity and prostacyclin synthase activity were measured initially, and after 2 and 4 weeks of treatment.
Results
In the nine participants with type 1 diabetes, treatment had no effect on any of the three indicators used to assess hyperglycaemia. However, treatment with benfotiamine plus α-lipoic acid completely normalised increased AGE formation, reduced increased monocyte hexosamine-modified proteins by 40% and normalised the 70% decrease in prostacyclin synthase activity from 1,709 ± 586 pg/ml 6-keto-prostaglandin F1α to 4,696 ± 533 pg/ml.
Conclusions/interpretation
These results show that the previously demonstrated beneficial effects of these agents on complication-causing pathways in rodent models of diabetic complications also occur in humans with type 1 diabetes.
Trial registration: NCT00703989
Funding: Juvenile Diabetes Research Foundation grant 8-2003-784 and GCRC grant MO1-RR12248.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benfotiamine blocks three major pathways of hyperglycaemic damage and prevents diabetic retinopathy and incipient nephropathy in experimental models [1, 2]. In cultured vascular cells, it also reduces aldose reductase gene expression and activity, as well as sorbitol levels [3]. It does so by activating the enzyme transketolase. α-Lipoic acid, a potent antioxidant, has also been reported to reduce diabetic microvascular and macrovascular complications in animal models [4, 5]. To determine whether benfotiamine in combination with α-lipoic acid would normalise markers of reactive oxygen species-induced pathways of complications in humans, we performed a pilot study in participants with type 1 diabetes using one daily dose of benfotiamine in combination with α-lipoic acid.
Methods
After protocol approval by the Committee on Clinical Investigations at the Albert Einstein College of Medicine, men with type 1 diabetes and matched healthy controls were recruited. Participant criteria included (in diabetic participants) diabetes duration between 0 and 15 years, current insulin therapy and no evidence of proliferative retinopathy, microalbuminuria, symptomatic diabetic neuropathy or cardiovascular disease. Participants taking any medications and those with a history of smoking were excluded. Age of enrolled participants was 28.9 ± 8.6 years, duration of diabetes was 25.5 ± 7.9 years and BMI was 20.3 ± 3.1 kg/m2. All participants gave written informed consent.
The glycaemic status of study patients was assessed by measuring baseline values of HbA1c, fructosamine and fasting plasma glucose. Mean HbA1c was 8.7 ± 0.7%, mean fructosamine was 421 ± 29 μmol/l (normal range 174–286 μmol/l) and mean fasting blood glucose was 11 ± 0.49 mmol/l.
At day 0, levels of markers of two benfotiamine-sensitive pathways were determined in participants: (1) intracellular AGE formation, as reflected by a marker of increased intracellular methylgloxal adducts in endothelial cells, angiopoietin 2 [6], and (2) hexosamine pathway activity, measured by determination of N-acetylglucosamine-modified protein in circulating monocytes [7]. Protein kinase C (PKC) activity in circulating monocytes could not be measured because the amount of blood required exceeded that approved by the Committee on Clinical Investigations. Serum levels of 6-keto-prostaglandin F (PGF)1α, a stable product produced by the non-enzymatic hydration of the anti-atherogenic mediator prostacyclin, were also determined [8]. Participants then took benfotiamine (300 mg twice a day; Advanced Orthomolecular Research, Calgary, AB, Canada), together with slow-release α-lipoic acid (600 mg twice a day; MRI, San Francisco, CA, USA) for 28 days. Blood was obtained at days 0, 15 and 28.
Data were analysed using one-factor analysis of variance to compare the means of all the groups. The Tukey–Kramer multiple comparisons procedure was used to determine which pairs of means were different.
Results
An initial study was performed with benfotiamine alone to determine whether the selected dose was sufficient to activate transketolase. In circulating monocytes, the dose of benfotiamine increased transketolase activity by twofold to threefold within 2 weeks (1.45 ± 0.17 vs 3.49 ± 0.22 nmol min−1 [mg protein]−1, mean ± SEM), an effect similar to that observed in long-term diabetic rat models.
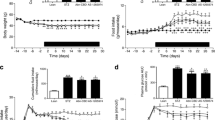
Following this, we examined the effect of combined treatment on angiopoietin-2, a marker of increased intracellular methylgloxal adducts in endothelial cells. Type 1 diabetes was associated with a 1.8-fold increase in circulating angiopoietin-2 levels (Fig. 1a). Treatment with benfotiamine plus α-lipoic acid completely normalised angiopoietin-2 levels by 2 weeks (2,416 ± 312 vs 1,062 ± 176 pg/ml, mean ± SEM). Treatment had no effect on any of the three variables used to assess hyperglycaemia in this study (data not shown).
a Angiopoietin-2 levels in serum of non-diabetic and type 1 diabetic participants before and during treatment with benfotiamine and α-lipoic acid. b Intracellular N-acetylglucosamine-modified protein (GlcNAc) in monocytes from non-diabetic and type 1 diabetic participants before and during treatment as above (a). c 6-keto-PGF1α levels in serum of non-diabetic and type 1 diabetic participants before and during same treatment (a). Non-diabetic group n = 12; type 1 diabetic group n = 9; **p < 0.01 compared with control; †p < 0.01 compared with week 0. AU, arbitrary units
Next, we examined the effect of combined treatment on hexosamine pathway activity by measuring total N-acetylglucosamine-modified proteins in circulating monocytes (Fig. 1b). Type 1 diabetes was associated with a 2.8-fold increase in hexosamine pathway activity (3,838 ± 765 vs 1,380 ± 616 arbitrary units, mean ± SEM). Two weeks of combined benfotiamine and lipoic acid treatment reduced this value by 40%.
Finally, type 1 diabetes was associated with a 70% decrease in activity of the critical endothelial anti-atherogenic enzyme prostacyclin synthase from 5,775 ± 294 pg/ml 6-keto-PGF1α, (mean ± SEM) to 1,709 ± 586 pg/ml 6-keto-PGF1α (mean ± SEM) (Fig. 1c). Treatment with benfotiamine plus α-lipoic acid normalised 6-keto-PGF1α activity by 4 weeks (4,696 ± 533 pg/ml 6-keto-PGF1α, mean ± SEM).
Discussion
Increased hyperglycaemia-induced superoxide causes glycolytic intermediates to be shunted into the major pathways of hyperglycaemic damage [9]. These intermediates, which activate intracellular AGE formation, the hexosamine pathway and PKC, are also the final products of the transketolase reaction. Because of this, increasing transketolase activity via benfotiamine blocks these complications-causing pathways. Although the damaging pathways inhibited by benfotiamine have been a major focus of complications research, it is important to recognise that excess superoxide can damage vascular cells without involvement of any of these pathways. An important example is the oxidative inactivation of prostacyclin synthase, a critical anti-atherosclerosis endothelial enzyme [10]. For this reason, we combined the antioxidant α-lipoic acid with benfotiamine [4].
In this pilot study we report that treatment with oral benfotiamine plus α-lipoic acid normalises several complications-causing pathways in patients with type 1 diabetes. The incomplete normalisation of the hexosamine pathway in monocytes, which contrasts with its complete normalisation in rat retina [1], may reflect differential accumulation of benfotiamine in different cell types or a slow turnover of monocyte intracellular proteins. Together, these results show that the beneficial effects of these agents on these pathways, as seen in rodent models of diabetic complications [1], also occur in humans with type 1 diabetes. Replication of these results in a much larger study population after optimisation of benfotiamine and α-lipoic acid doses will be necessary in order to determine whether this treatment may help prevent diabetic retinopathy and nephropathy in human patients, as it does in diabetic animal models.
Abbreviations
- PGF:
-
prostaglandin F
- PKC:
-
protein kinase C
References
Hammes H-P, Du X, Edelstein D et al (2003) Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9:294–299
Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ (2003) Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes 52:2110–2120
Berrone E, Beltramo E, Solimine C, Ape AU, Porta M (2006) Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem 281:9307–9313
Lin J, Bierhaus A, Bugert P et al (2006) Effect of R-(+)-alpha-lipoic acid on experimental diabetic retinopathy. Diabetologia 49:1089–1096
Yi X, Maeda N (2006) alpha-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes 55:2238–2244
Yao D, Taguchi T, Matsumura T et al (2007) High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem 282:31038–31045
Zachara NE, Hart GW, Cole RN, Gao Y (2002) Detection and analysis of proteins modified by O-linked N-acetylglucosamine. In: Current protocols in protein science. John Wiley, New York, chapter 17, unit 17.6
Du X, Edelstein D, Obici S, Higham N, Zou M-H, Brownlee M (2006) Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest 116:1071–1080
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Zou MH, Shi C, Cohen RA (2002) High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes 51:198–203
Acknowledgements
This work was supported by Juvenile Diabetes Research Foundation grant 8-2003-784 and General Clinical Research Centre grant MO1-RR12248.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Edelstein died during the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Du, X., Edelstein, D. & Brownlee, M. Oral benfotiamine plus α-lipoic acid normalises complication-causing pathways in type 1 diabetes. Diabetologia 51, 1930–1932 (2008). https://doi.org/10.1007/s00125-008-1100-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-008-1100-2