Abstract
Aims/hypothesis
Opening of ATP-sensitive potassium (KATP) channels during myocardial ischaemia shortens action potential duration and is believed to be an adaptive, energy-sparing response. Thiazolidinedione drugs block KATP channels in non-cardiac cells in vitro. This study determined whether thiazolidinedione drugs block cardiac KATP channels in vivo.
Methods
Experiments in 68 anaesthetised pigs determined: (1) effects of inert vehicle, troglitazone (10 mg/kg i.v.) or rosiglitazone (0.1 or 1.0 mg/kg i.v.) on epicardial monophasic action potential (MAP) during 90 min low-flow ischaemia; (2) effects of troglitazone, rosiglitazone or pioglitazone (1 mg/kg i.v.) on response of MAP to intracoronary infusion of a KATP channel opener, levcromakalim; and (3) effects of inert vehicle, rosiglitazone (1 mg/kg i.v.) or the sarcolemmal KATP blocker HMR-1098 on time to onset of ventricular fibrillation following complete coronary occlusion.
Results
With vehicle, epicardial MAP shortened by 44 ± 9 ms during ischaemia. This effect was attenuated to 12 ± 8 ms with troglitazone and 6 ± 6 ms with rosiglitazone (p < 0.01 for both vs vehicle), suggesting KATP blockade. Intracoronary levcromakalim shortened MAP by 38 ± 10 ms, an effect attenuated to 12 ± 8, 13 ± 4 and 9 ± 5 ms during co-treatment with troglitazone, rosiglitazone or pioglitazone (p < 0.05 for each), confirming KATP blockade. During coronary occlusion, median time to ventricular fibrillation was 29 min in pigs treated with vehicle and 6 min in pigs treated with rosiglitazone or HMR-1098 (p < 0.05 for both vs vehicle), indicating that KATP blockade promotes ischaemic ventricular fibrillation in this model.
Conclusions/interpretation
Thiazolidinedione drugs block cardiac KATP channels at clinically relevant doses and promote onset of ventricular fibrillation during severe ischaemia.
Similar content being viewed by others
Introduction
Cardiac ATP-sensitive potassium (KATP) channels open during ischaemia, causing shortening of action potential duration [1]. This is generally believed to be an adaptive, energy-sparing response [2, 3]. Opening of cardiac KATP channels in ischaemia also stabilises resting membrane potential and may therefore have anti-arrhythmic effects [4–6]. Conversely, pharmacological blockade or genetic ablation of KATP channels may be harmful, promoting ischaemic and catecholamine-induced arrhythmias and abolishing ischaemic preconditioning [7–11]. However, findings are divided in this regard. When KATP channels open during ischaemia, resultant dispersion of refractoriness and slowing of conduction could predispose to re-entrant arrhythmias; by preventing these effects, KATP channel blockade could be anti-arrhythmic and protective. In fact, some studies have indicated that KATP channel blockers reduce the propensity for ischaemic arrhythmias [12, 13], while KATP channel openers facilitate induction of ventricular fibrillation [14–16].
Many patients with type 2 diabetes are treated with thiazolidinedione drugs for their insulin sensitising effects. In addition, thiazolidinediones exert anti-inflammatory and anti-proliferative effects in experimental studies and favourably modify serum lipoproteins, circulating inflammatory markers and progression of atherosclerosis as identified by vascular imaging in clinical investigations. On this basis, one might expect dramatic improvement in clinical outcomes among patients treated with thiazolidinediones. However, these expectations have not been fulfilled. While clinical outcomes with pioglitazone tended towards being favourable in the PROACTIVE trial [17] and were favourable in a meta-analysis [18], a meta-analysis of trials with rosiglitazone indicated increased cardiovascular morbidity and mortality rates with that agent [19]. Could adverse cardiovascular effects of thiazolidinedione drugs diminish or negate their favourable metabolic effects? One potential explanation is that these agents precipitate or exacerbate fluid retention and congestive heart failure. Another possibility is that they exert direct adverse cardiac effects. Other compounds in the class, including troglitazone and englitazone, block KATP channels in a variety of non-cardiac cells in vitro [20–23]; however, it is not known whether thiazolidinediones exert similar effects in the heart.
The goal of the present study was to determine whether thiazolidinedione drugs interact with cardiac KATP channels and affect the cardiac action potential and rhythm in vivo, using a porcine model of myocardial ischaemia.
Methods
Anaesthesia, surgery and instrumentation
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Anaesthesia and instrumentation followed previously published methods [24–27]. Domestic pigs (n = 68) of either sex weighing 24 ± 1 kg were studied in three sets of experiments. Pigs were sedated with ketamine (25 mg/kg, intramuscular), anaesthetised with alpha α-chloralose (100 mg/kg i.v. induction, 20–30 mg kg−1 h−1 i.v. maintenance), intubated and mechanically ventilated with oxygen-enriched air, and wrapped in a recirculating warm-water blanket to maintain body temperature. Propranolol (1 mg/kg i.v.) and atropine (0.2 mg/kg i.v.) were given to prevent changes in autonomic tone and spontaneous heart rate during experiments. A carotid artery and a jugular vein were cannulated. Bipolar pacing electrodes were affixed to the left atrial appendage and used to maintain heart rate at 10 beats per min above the spontaneous rate. A micromanometer catheter measured left ventricular pressure. For ischaemia experiments, the proximal portion of the left anterior descending coronary artery (LAD) was instrumented with an ultrasonic flow probe (Transonic Systems, Ithaca, NY, USA) and adjustable hydraulic occluder (In Vivo Metrics, Healdsburg, CA, USA). In levcromakalim experiments, the LAD was instrumented with an ultrasonic flow probe and a 23-gauge stainless steel cannula for drug infusion. Needle electrodes were attached to each limb to record the six standard limb leads of the surface electrocardiogram. Monophasic action potentials (MAPs) [28] were recorded from the left ventricular epicardial surface with an electrode (model 501; EP Technologies, Mountain View, CA, USA) that maintains contact pressure by a spring tensioning mechanism. MAP recordings were pre-amplified with a high-input impedance, direct current-coupled differential amplifier, digitised at 10 kHz and recorded with an electrophysiology data system (EPACE; Fischer Imaging, Denver, CO, USA). MAPs were recorded from anterior and posterolateral free walls of the left ventricle. MAP duration was measured from the point of initial upward deflection to the point of 90% recovery from peak of phase 0 depolarisation. MAP rise time was calculated as the time from 10 to 90% of peak phase 0 depolarisation. Criteria for acceptance of MAP data for analysis were: (1) constant morphology and resting potential before and after measurement; and (2) amplitude of phase 2 depolarisation exceeding 10 mV.
Effect of troglitazone and rosiglitazone on action potential during ischaemia and reperfusion
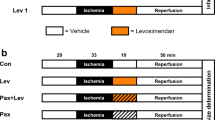
We studied 30 pigs in a low-flow regional myocardial ischaemia and reperfusion experiment. The experimental design is diagrammed in Fig. 1. Baseline (pre-treatment) measurements of haemodynamics, electrocardiogram and MAP were made. MAP was recorded in the central portion of the area to be rendered ischaemic, identified as myocardium subtended by diagonal branches with origins distal to the LAD occluder; the boundaries of the ischaemic region were subsequently confirmed by visible akinesis during coronary constriction. MAP was also recorded in the non-ischaemic posterolateral region of the left ventricle. Pigs were then treated with intravenous troglitazone (n = 11), intravenous rosiglitazone (n = 8) or inert vehicle (n = 11), each beginning 1 h before ischaemia and continuing through reperfusion. Crystalline troglitazone (Sankyo Pharmaceutical Research, Tokyo, Japan) was dissolved in a 50% (v/v) aqueous solution of polyethylene glycol-400 and 0.15 mol/l NaHCO3 to a concentration of 10 mg/ml (25 mmol/l) and administered as 10 mg/kg i.v. over 10 min followed by 5 mg kg−1 h−1 i.v. for the remainder of the experiment. This dose of troglitazone results in a plasma troglitazone concentration of 5 ± 1 μg/ml in pigs [26], similar to that achieved in prior clinical use of this drug [29]. Rosiglitazone potassium salt (Cayman Chemical, Ann Arbor, MI, USA) was dissolved in DSMO (1:10, w/v) and diluted with distilled water to a final concentration of 2 mg/ml. Four pigs were treated with a dose of 0.1 mg/kg i.v. and four other pigs with 1.0 mg/kg i.v., administered over 10 min. In the vehicle group, polyethylene glycol was used in eight pigs and DMSO in three pigs. In each treatment group, a second set of measurements of haemodynamics, electrocardiogram and MAP was obtained 1 h after initiation of treatment, just before the onset of ischaemia (designated ‘pre-ischaemic treatment’). The LAD occluder was then gradually constricted using a microsyringe to reduce coronary flow rate to 50% of baseline (±1 ml/min) and maintained at this level for 90 min. This degree of ischaemia results in approximately 80% reduction of subendocardial left ventricular blood flow and approximately 55% reduction of transmural left ventricular blood flow without significant infarction in pigs [24–27, 30]. Measurements were repeated at 15, 30, 60 and 90 min of ischaemia. The LAD occluder was then released and measurements obtained at 45 and 90 min of reperfusion.
Effect of thiazolidinediones on action potential shortening induced by the KATP channel-opener levcromakalim
We studied 19 pigs in these experiments. Levcromakalim (Sigma Aldrich, St Louis, MO, USA), the active enantiomer of the KATP channel opener cromakalim, was dissolved in DMSO (1:50, w/v) and diluted with distilled water to final concentration of 150 μg/ml. Levcromakalim was chosen for its selectivity and potency as a KATP channel opener and its short half-life of approximately 5 min [31, 32]. The experimental design is diagrammed in Fig. 2. In six pigs, the MAP response to two doses of levcromakalim (1 and 2 μg kg−1 min−1 via intracoronary infusion, 10 min each) was evaluated before and after treatment with troglitazone (Fig. 2a). Troglitazone treatment (10 mg/kg i.v. over 10 min, followed by 5 mg kg−1 h−1 i.v.) was initiated 45 min after the first application of levcromakalim and 1 h before the second application of levcromakalim. In six other pigs, MAP response to a single dose of levcromakalim (1 μg kg−1 min−1 intracoronary) was evaluated before and after two doses of rosiglitazone (0.1 and 1.0 mg/kg; Fig. 2b). Rosiglitazone 0.1 mg/kg i.v. was administered 45 min after the first application of levcromakalim and 1 h before the second application. An additional dose of rosiglitazone 0.9 mg/kg i.v. (to achieve a cumulative dose of 1.0 mg/kg) was administered 45 min after the second application of levcromakalim and 1 h before the third application. In five other pigs, MAP response to levcromakalim (1 μg kg−1 min−1) was evaluated before and after administration of pioglitazone (Fig. 2c). Pioglitazone HCl (Molcan, Richmond Hill, ON, Canada) was dissolved in polyethylene glycol 400, diluted with an equal volume of 0.15 mol/l NaHCO3 to a final concentration of 1 mg/ml and administered at a dose of 1 mg/kg i.v. over 10 min.
Schematic representation of protocol for assessing effects of troglitazone (a), rosiglitazone (b) and pioglitazone (c) on response of MAP to intracoronary infusion of levcromakalim (KATP channel opener). One dose of troglitazone was evaluated during treatment with two doses of levcromakalim. Two doses of rosiglitazone and one dose of pioglitazone were evaluated during treatment with one dose of levcromakalim
In two additional pigs, levcromakalim infusion was repeated twice without thiazolidinedione treatment, allowing 1 h between the end of the first and the beginning of the second infusion. Haemodynamics and MAP rapidly returned to baseline after discontinuing the first infusion; responses to the second infusion were nearly identical to the first (data not shown). Therefore, it was deemed appropriate to administer sequential infusions of levcromakalim before and after troglitazone or rosiglitazone, allowing comparison of responses with and without thiazolidinedione in the same pigs.
Effect of rosiglitazone on time of onset of ischaemic ventricular fibrillation
Pigs were pretreated with rosiglitazone 1 mg/kg i.v., the selective sarcolemmal KATP blocker HMR-1098 (3 mg/kg i.v. load, 1 mg kg−1 h−1 i.v. infusion) [33] or inert vehicle (n = 6–7 each group). At 1 h after pre-treatment, the mid-portion of the left anterior coronary artery was completely occluded for up to 90 min and the time from coronary occlusion to onset of ventricular fibrillation was observed.
Data analysis
Data are expressed as mean ± SEM. MAP response to ischaemia and reperfusion was compared between groups (troglitazone or rosiglitazone and vehicle) using two-way repeated measures analysis of variance (factors treatment and time). A significant interaction between treatment and time indicates that treatment with thiazolidinedione modifies the overall time course of MAP duration in response to ischaemia and reperfusion. Post hoc between-group comparisons at individual time points were performed with unpaired t tests. Within-group comparisons between baseline and subsequent time points were performed with Tukey’s procedure. The effect of troglitazone, rosiglitazone, or pioglitazone on MAP shortening in response to levcromakalim was assessed by two-way repeated measures analysis of variance with post hoc paired comparisons. Difference between groups in the incidence of ventricular fibrillation during low-flow ischaemia was evaluated by χ 2 analysis. The effect of rosiglitazone or HMR-1098, compared with vehicle, on time of onset of ventricular fibrillation during complete coronary occlusion was determined by rank sum test. Statistical significance was accepted at the level of p < 0.05.
Results
Low-flow ischaemia-reperfusion experiment
There was no significant difference among groups in the incidence of ventricular fibrillation during low-flow ischaemia or reperfusion. Fibrillation occurred in four pigs treated with vehicle, four pigs treated with troglitazone and in none of the pigs treated with rosiglitazone (p = 0.14 for comparison of the three groups, p = 0.63 for comparison of the combined thiazolidinedione groups with the vehicle group). Pigs that fibrillated were not resuscitated and data are included up to the last measurement point in the protocol preceding death.
Haemodynamic and MAP data for the three groups of pigs are shown in Tables 1 and 2. In the absence of ischaemia, neither troglitazone nor rosiglitazone had a significant effect on MAP duration, consistent with the fact that KATP channels are predominantly closed under non-ischaemic conditions.
As expected, pigs in the vehicle group demonstrated shortening of MAP duration during ischaemia (Fig. 3a,d). The greatest degree of MAP shortening (44 ± 9 ms) was observed early in ischaemia. MAP duration gradually recovered during late ischaemia and returned to baseline by the end of reperfusion. Shortening of MAP duration is the expected response to ischaemia, due primarily to opening of KATP channels [1]. The magnitude of observed changes was similar to those demonstrated by other investigators in dogs and pigs [4, 34].
a Representative epicardial MAP recordings at baseline (continuous line), at 15 min of low-flow myocardial ischaemia (dotted line) and at 90 min of reperfusion (dashed line) in a pig treated with inert vehicle. For clarity of comparison, MAP amplitude during ischaemia and reperfusion has been normalised to phase 0 amplitude at baseline. MAP duration shortened during ischaemia and recovered with reperfusion. b Representative MAP recordings at baseline, 15 min ischemia and 90 min reperfusion in a pig treated with troglitazone (10 mg/kg i.v. loading dose followed by 5 mg kg−1 h−1 i.v. infusion). c Representative MAP recordings at baseline, 15 min ischemia and 90 min reperfusion in a pig treated with rosiglitazone (1 mg/kg i.v.). Troglitazone and rosiglitazone markedly attenuated the degree of MAP shortening during ischaemia. d Group data for response of MAP to ischaemia (Isc) and reperfusion (Rep) in the presence of vehicle (white bars, n = 11), troglitazone (black bars, n = 11) and rosiglitazone (hatched bars, n = 8). In the rosiglitazone group, four pigs were treated with a dose of 0.1 mg/kg and four pigs were treated with 1.0 mg/kg; effects of both doses were similar and data are combined. Data are mean ± SEM. As in the exemplary recordings, troglitazone and rosiglitazone attenuated the degree of MAP shortening that occurred during ischaemia. The overall time course of response differed (p < 0.01) between the vehicle vs troglitazone and vehicle vs rosiglitazone groups. Post hoc analysis indicated that MAP duration differed (*p < 0.05) between the vehicle and the troglitazone or rosiglitazone groups at the individual time points indicated
Pigs treated with troglitazone or rosiglitazone demonstrated much less shortening of MAP during ischaemia than pigs treated with vehicle (p < 0.01 for difference in overall time course) (Table 2, Fig. 3b–d). For example, at 15 min ischaemia MAP shortened by 6 ± 6 ms in the troglitazone group and 2 ± 7 ms in the rosiglitazone group, both significantly less (p < 0.01) than 44 ± 9 ms in the vehicle group. At 30 min ischaemia, MAP shortened by 12 ± 8 ms in the troglitazone group and 6 ± 6 ms in the rosiglitazone group, compared with 25 ± 8 ms in the vehicle group. There was no significant difference in the effects of rosiglitazone 0.1 or 1.0 mg/kg on MAP duration during ischaemia, indicating that the effect was near-maximal at the lower, clinically relevant dose. Attenuation of MAP shortening by troglitazone and rosiglitazone during ischaemia suggested that these agents act as cardiac KATP blockers and provided the motivation for our second set of experiments, in which the effects of these agents were evaluated in response to treatment with a pharmacological KATP channel opener, levcromakalim.
The rise time of phase 0 of the MAP is related to conduction velocity [28], which generally slows with ischaemia. MAP rise time was prolonged slightly with ischaemia in the vehicle and troglitazone groups, but changes in this variable did not reach statistical significance within any group, nor did rise time differ significantly among the three groups (Table 2). QRS duration measured from surface ECG averaged 70 ms at baseline and was not affected by ischaemia or by treatment with troglitazone or rosiglitazone. In most experiments, QT interval could not be measured accurately from the surface electrocardiogram because of superimposition of the atrial pacing stimulus and/or P-wave on the T-wave at the prevailing heart rate.
Levcromakalim experiments
Levcromakalim produced dose-dependent shortening of MAP duration (Table 3), consistent with its action as a KATP channel opener. Levcromakalim also lowered systolic blood pressure and increased coronary blood flow rate. Upon discontinuation of levcromakalim, all measures returned to baseline within 5 min.
The MAP shortening produced by levcromakalim was markedly attenuated by pre-treatment with troglitazone (Fig. 4), rosiglitazone (Fig. 5) or pioglitazone (Fig. 6; p < 0.05 for each). Levcromakalim 1 μg kg−1 min−1 shortened MAP by 32 ± 19 ms in the absence of troglitazone, compared with 12 ± 8 ms in the presence of troglitazone. Levcromakalim 2 μg kg−1 min−1 shortened MAP by 76 ± 23 ms in the absence of troglitazone, compared with 22 ± 8 ms in the presence of troglitazone. Similarly, levcromakalim 1 μg kg−1 min−1 shortened MAP by 41 ± 14 ms in the absence of rosiglitazone, compared with 16 ± 2 ms in the presence of rosiglitazone 0.1 mg/kg and 11 ± 5 ms in the presence of rosiglitazone 1.0 mg/kg. As in the ischaemia experiments, a near-maximal effect of rosiglitazone on MAP duration was observed at the lower, clinically relevant dose of 0.1 mg/kg. Levcromakalim 1 μg kg−1 min−1 shortened MAP by 38 ± 7 ms in the absence of pioglitazone, compared with 9 ± 5 ms in the presence of pioglitazone 1.0 mg/kg. Despite the effects of each thiazolidinedione on MAP shortening in response to levcromakalim, there was no discernible effect of thiazolidinedione treatment on the decrease of systolic blood pressure or increase of coronary blood flow in response to levcromakalim (Table 3).
a Representative MAP recordings obtained at baseline (continuous line), during infusion of the KATP channel opener levcromakalim (2 μg kg−1 min−1, intracoronary; dotted line) and during intracoronary infusion of levcromakalim after treatment with troglitazone (10 mg/kg i.v. loading dose followed by 5 mg kg−1 h−1 i.v. infusion; dashed line). To facilitate comparison, recordings during drug treatment have been normalised to phase 0 amplitude at baseline. Troglitazone attenuated the degree of MAP shortening that otherwise occurs with levcromakalim. b Group data (n = 6, mean ± SEM). In the absence of troglitazone (control, white bars), levcromakalim produced dose-dependent shortening of MAP duration; this effect was attenuated by troglitazone (black bars; p < 0.05 for effect of troglitazone on overall levcromakalim dose–response relationship). *p < 0.05 for difference between troglitazone and control at 2 μg kg−1 min−1 dose of levcromakalim
a Representative MAP recordings obtained at baseline (continuous line), during infusion of the KATP channel opener levcromakalim (1 μg kg−1 min−1, intracoronary, dotted line) and during intracoronary infusion of levcromakalim after treatment with rosiglitazone (0.1 mg/kg i.v.; dashed line). Recordings during drug treatment have been normalised to phase 0 amplitude at baseline. Rosiglitazone attenuated the degree of MAP shortening that otherwise occurs with levcromakalim. b Group data (n = 6, mean ± SEM). Rosiglitazone produced a dose-dependent attenuation of the degree of MAP shortening that otherwise occurs with levcromakalim. *p < 0.05 for difference from control
a Representative MAP recordings obtained at baseline (continuous line), during infusion of the KATP channel opener levcromakalim (1 μg kg−1 min−1, intracoronary, dotted line) and during intracoronary infusion of levcromakalim after treatment with pioglitazone (1 mg/kg i.v.; dashed line). Recordings have been normalised to peak phase 0 amplitude at baseline. Pioglitazone attenuated the degree of MAP shortening that otherwise occurs with levcromakalim. b Group data (n = 5, mean ± SEM). In the absence of pioglitazone (control), levcromakalim shortened MAP duration; this effect was significantly attenuated by pioglitazone (p < 0.05)
Effect of rosiglitazone on time of onset of ischaemic ventricular fibrillation during coronary occlusion
Six of seven pigs treated with vehicle and all pigs treated with rosiglitazone or HMR-1098 (n = 6 each) developed ventricular fibrillation during complete coronary occlusion. Fibrillation occurred at a median 6 min of ischaemia in the rosiglitazone group and 6 min in the HMR-1098 group, compared with 29 min in the vehicle group (p < 0.05 for both drugs compared with vehicle; Fig. 7). A time of 90 min was assigned to one pig in the vehicle group that had not fibrillated at 90 min of coronary occlusion.
Discussion
This study demonstrates that the thiazolidinedione drugs troglitazone, rosiglitazone and pioglitazone block the cardiac sarcolemmal KATP channel in vivo. The evidence for such an effect is that the drugs markedly attenuated the degree of action potential shortening induced by myocardial ischaemia or by local administration of a pharmacological KATP channel opener. Because these effects were observed shortly after administration of the thiazolidinedione drugs, it is unlikely that they were transcriptionally mediated via the nuclear receptor peroxisome proliferator-activated receptor (PPAR)-γ. Rather, they more probably reflect direct interaction of the drugs with the sarcolemmal K ATP channel. The present study is consistent with previous demonstrations that thiazolidinediones block KATP channels in non-cardiac cells in culture [20–23], but is the first to demonstrate an analogous effect in the heart in vivo.
During low-flow ischaemia and reperfusion, when a minority of pigs develop ventricular fibrillation, we observed no significant effect of thiazolidinedione treatment on the incidence of ventricular fibrillation. In a previous study using the same dose of troglitazone and the same duration and severity of low-flow ischaemia, we observed an excess of ventricular fibrillation among troglitazone-treated pigs [26]. However, comparison of that study with the present one may be confounded by the fact that pigs in the prior study were co-treated with lidocaine, which can be pro-fibrillatory in pigs [35] and might interact with troglitazone.
In contrast, during complete coronary occlusion, when nearly all pigs develop ventricular fibrillation, we found that treatment with rosiglitazone significantly shortened the time to onset of fibrillation. A similar pro-fibrillatory effect was observed after treatment with the selective sarcolemmal KATP blocker, HMR-1098, supporting the notion that KATP blockade is the mechanism of pro-arrhythmia with rosiglitazone. A previous study in rats demonstrated that rosiglitazone increased late mortality after myocardial infarction, presumed due to an increased risk of sudden cardiac death [36].
The opening of KATP channels that ordinarily occurs during myocardial ischaemia has the potential to produce pro-arrhythmic or anti-arrhythmic effects [2, 37]. Increased dispersion of refractoriness and/or slowing of conduction by the KATP current may predispose to re-entrant arrhythmias, while stabilisation of resting membrane potential may suppress automatic or triggered arrhythmias. Conversely, blockade of KATP channels during ischaemia could be anti-arrhythmic if re-entrant arrhythmias are prevented or pro-arrhythmic if automatic or triggered arrhythmias are facilitated [37]. It is possible that these opposing actions resulted in the neutral effect of thiazolidinedione treatment on ventricular fibrillation observed during low-flow ischaemia, but that predominance of the latter action accounts for the promotion of ventricular fibrillation by rosiglitazone (and HMR-1098) observed during complete coronary occlusion. Consistent with the current observations, mice with genetic ablation of cardiac KATP channels demonstrate increased susceptibility to lethal ventricular arrhythmias [8, 11], while pharmacological KATP openers have been shown to extend time to ventricular fibrillation and decrease incidence of ventricular fibrillation during coronary occlusion in pigs and dogs [5, 6]. However, we cannot exclude the possibility that unmeasured effects of thiazolidinediones on ion channels other than KATP [38–41] also influenced the development of ischaemic arrhythmias in the present study.
In contrast to the present results, prior studies in pigs [13], dogs [33, 42], rats [43] and rabbits [44] have shown a reduction in ischaemic ventricular fibrillation with the sarcolemmal KATP blocker HMR 1098 or its congener HMR 1883. The effects of KATP blockade on ischaemic arrhythmias may depend upon the specific conditions of each experimental model [2]. For example, differences between the prior porcine study with HMR 1883 [13] and the present study include the duration of coronary occlusion (20 vs 90 min), the use of autonomic blockade in the present study and the type of anaesthesia (pentobarbital versus α-chloralose). Barbiturate anaesthetics may independently block mitochondrial [45] and sarcolemmal [46] KATP channels. In the prior porcine study [13], it should also be noted that while HMR 1883 reduced ventricular fibrillation during ischaemia, 5 of 12 treated pigs developed ventricular fibrillation during the first few minutes of reperfusion.
In the present study, blockade of cardiac KATP channels by thiazolidinediones was demonstrated at clinically relevant doses of the drugs. The dose of troglitazone used in this study results in an average plasma troglitazone concentration of 5 μg/ml in pigs [26], similar to 3 μg/ml measured in patients after a single oral dose of 600 mg [29]. Similarly, rosiglitazone 0.1 mg/kg and pioglitazone 1.0 mg/kg are comparable to typical daily oral doses of these agents in clinical use. We allowed 1 h between the administration of thiazolidinedione drugs and the beginning of ischaemia or levcromakalim infusion. At 1 h following intravenous administration of troglitazone or rosiglitazone, pharmacokinetics are similar to those observed after oral administration of an equal dose [47, 48].
Under normal conditions, sarcolemmal KATP channels are predominantly closed; therefore, it is not surprising that thiazolidinediones, acting as KATP channel blockers, do not significantly affect MAP in the absence of ischaemia. During ischaemia, the attenuation of MAP shortening produced by troglitazone and rosiglitazone is similar to that produced by the prototypical KATP blocker, glibenclamide, in pigs [9].
We found that troglitazone, rosiglitazone and pioglitazone each abrogated shortening of MAP to a similar degree in response to a KATP opener, levcromakalim. A previous in vitro study indicated that reconstituted KATP channels in COS-1 cells are blocked by troglitazone, but not by pioglitazone [23]. The discrepancy between that study and the current data highlights the importance of translational in vivo studies in this area.
The current data may provide clues regarding the type of interaction between thiazolidinediones and KATP channels. Each tested thiazolidinedione compound attenuated MAP shortening in response to levcromakalim, but did not attenuate the decrease in systolic blood pressure or the increase in coronary blood flow produced by levcromakalim. These observations indicate differential effects of thiazolidinediones on cardiac and vascular smooth muscle KATP channels. The former are composed of Kir6.2 and SUR2A subunits, while the latter are composed primarily of Kir6.1 and SUR2B subunits. Thus, the present findings suggest that thiazolidinediones interact with either Kir6.2 or SUR2A subunits, but not with Kir6.1 or SUR2B. An interaction of thiazolidinediones with Kir6.2 is supported by studies in cell culture [22, 23] and would contrast with the actions of sulfonylurea KATP blockers, which interact with SUR subunits.
From a metabolic and functional standpoint, opening of cardiac KATP channels during ischaemia is generally considered to be adaptive and protective. By promoting repolarisation and shortening action potential duration, calcium entry is reduced and metabolic energy is conserved [2, 3]. These adaptive effects of K ATP channel opening lead to the prediction that blockade of K ATP channels during myocardial ischaemia would impair contractile recovery. In fact, some in vitro studies have shown that glibenclamide impairs contractile recovery after simulated ischaemia [49]. However, evidence for an adverse effect of KATP blockade on contractile recovery in vivo is less convincing. KATP blockade with glibenclamide abolishes protection by ischaemic preconditioning, but has a neutral effect on post-ischaemic contractile function and infarct size in pigs when no preconditioning stimulus is applied [9]. HMR 1098 had no significant effects on contractility at baseline or during ischaemia in the canine heart [50]. Similarly, we previously found neutral effects of chronic rosiglitazone treatment [25] or acute troglitazone treatment [26] on recovery of contractile function after low-flow ischaemia without preconditioning in pigs. It remains an open question whether myocardial infarct size and protection by ischaemic preconditioning are altered by thiazolidinedione treatment. KATP blockers such as glibenclamide affect both sarcolemmal and mitochondrial channels. Using MAP duration as an indicator, we characterised effects of troglitazone, rosiglitazone and pioglitazone on the sarcolemmal KATP channel. The current data do not indicate whether thiazolidinedione drugs block mitochondrial KATP channels or whether they act upon other types of sarcolemmal ion channels [37–40].
In clinical practice, it remains uncertain whether treatment with thiazolidinedione drugs produces net clinical benefit or harm in patients with cardiac disease. The answer probably depends upon several interacting factors. Beyond effects on KATP channels, thiazolidinediones produce anti-inflammatory and metabolic effects related to PPAR-γ, as well as changes in systemic vascular resistance, cardiac output and plasma volume, each of which may influence the net effect of treatment. Nonetheless, the fact that the outcomes of clinical trials with thiazolidinediones have been less favourable than expected [17] or perhaps even unfavourable [19], despite clearly beneficial metabolic effects, raises a suspicion that countervailing, unfavourable mechanisms are also operative. It is possible that blockade of cardiac KATP channels is such a mechanism.
Abbreviations
- KATP :
-
ATP-sensitive potassium
- Kir:
-
potassium inward rectifying
- LAD:
-
left anterior descending coronary artery
- MAP:
-
monophasic action potential
- PPAR:
-
peroxisome proliferator-activated receptor
- SUR:
-
sulfonylurea receptor
References
Saito T, Sato T, Miki T, Seino S, Nakaya H (2005) Role of ATP-sensitive K+ channels in electrophysiological alterations during myocardial ischemia: a study using Kir6.2-null mice. Am J Physiol Heart Circ Physiol 288:H352–H357
Flagg TP, Nichols CG (2005) Sarcolemmal KATP channels: what do we really know? J Mol Cell Cardiol 39:61–70
Gross GJ, Peart JN (2003) KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol 285:H921–H930
D’Alonzo AJ, Darbenzio RB, Parham CS, Grover GJ (1992) Effects of intracoronary cromakalim on postischaemic contractile function and action potential duration. Cardiovasc Res 26:1046–1053
D’Alonzo AJ, Hess TA, Darbenzio RB, Sewter JC, Conder ML, McCullough JR (1994) Effects of cromakalim or pinacidil on pacing- and ischemia-induced ventricular fibrillation in the anesthetized pig. Basic Res Cardiol 89:163–176
Tanabe T, Aikawa M, Deguchi Y, Yoshioka K, Handa S (2000) Attenuation of KATP channel-opener induced shortening of repolarization by alpha-1-adrenoceptor during ischemia in canine heart. Cardiovasc Drugs Ther 14:283–294
Driamov S, Bellahcene M, Ziegler A et al (2004) Antiarrhythmic effect of ischemic preconditioning during low-flow ischemia. The role of bradykinin and sarcolemmal versus mitochondrial ATP-sensitive K+ channels. Basic Res Cardiol 99:299–308
Tong XY, Porter LM, Liu GX et al (2006) Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am J Physiol Heart Circ Physiol 291:H543–H551
Schulz R, Rose J, Heusch G (1994) Involvement of activation of ATP-dependent potassium channels in ischemic preconditioning in swine. Am J Physiol 267:H1341–H1352
Gumina RJ, Pucar D, Bast P et al (2003) Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol Heart Circ Physiol 284:H2106–H2113
Liu XK, Yamada S, Kane GC et al (2004) Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes 53(Suppl 3):S165–S168
Picard S, Rouet R, Ducouret P et al (1999) KATP channels and “border zone” arrhythmias: role of the repolarization dispersion between normal and ischaemic ventricular regions. Br J Pharmacol 127:1687–1695
Wirth KJ, Rosenstein B, Uhde J, Englert HC, Busch AE, Scholken BA (1999) ATP-sensitive potassium channel blocker HMR 1883 reduces mortality and ischemia-associated electrocardiographic changes in pigs with coronary occlusion. J Pharmacol Exp Ther 291:474–481
Wolk R, Cobbe SM, Kane KA, Hicks MN (1999) Relevance of inter- and intraventricular electrical dispersion to arrhythmogenesis in normal and ischaemic rabbit myocardium: a study with cromakalim, 5-hydroxydecanoate and glibenclamide. J Cardiovasc Pharmacol 33:323–334
Fischbach PS, White A, Barrett TD, Lucchesi BR (2004) Risk of ventricular proarrhythmia with selective opening of the myocardial sarcolemmal versus mitochondrial ATP-gated potassium channel. J Pharmacol Exp Ther 309:554–559
Yao Z, Gross GJ (1994) Effects of the KATP channel opener bimakalim on coronary blood flow, monophasic action potential duration, and infarct size in dogs. Circulation 89:1769–1775
Dormandy J, Charbonnel B, Eckland DJA et al (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366:1279–1289
Lincoff AM, Wolski K, Nicholls SJ, Nissen SE (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus. JAMA 298:1180–1188
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471
Lee K, Ibbotson T, Richardson PJ, Boden PR (1996) Inhibition of KATP channel activity by troglitazone in CRI-G1 insulin-secreting cells. Eur J Pharmacol 313:163–167
Lee K, Boden P (1997) Troglitazone inhibits type 2 KATP channel activity and depolarises tolbutamide-sensitive neurons in the rat ventromedial hypothalamus. Brain Res 751:165–168
McKay NG, Kinsella JM, Campbell CM, Ashford ML (2000) Sensitivity of Kir6.2-SUR1 currents, in the absence and presence of sodium azide, to the KATP channel inhibitors, ciclazindol and englitazone. Br J Pharmacol 130:857–866
Sunaga Y, Inagaki N, Gonoi T et al (1999) Troglitazone but not pioglitazone affects ATP-sensitive K+ channel activity. Eur J Pharmacol 381:71–76
Zhu P, Lu L, Xu Y, Greyson C, Schwartz GG (2000) Glucose-insulin-potassium preserves systolic and diastolic function in ischemia and reperfusion in pigs. Am J Physiol Heart Circ Physiol 278:H595–H603
Xu Y, Gen M, Lu L et al (2005) PPAR-γ activation fails to provide myocardial protection in ischemia and reperfusion in pigs. Am J Physiol Heart Circ Physiol 288:H1314–H1325
Xu Y, Lu L, Greyson C et al (2003) Deleterious effects of acute treatment with a peroxisome proliferator-activated receptor-γ activator in myocardial ischemia and reperfusion in pigs. Diabetes 52:1187–1194
Lu L, Xu Y, Greyson C, Ursell PC, Schwartz GG (1999) Nonelastic deformation of myocardium in low-flow ischemia and reperfusion: ultrastructure-function relations. J Mol Cell Cardiol 31:1157–1169
Franz MR (1991) Method and theory of monophasic action potential recording. Prog Cardiovasc Dis 33:347–368
Spencer CM, Markham A (1997) Troglitazone. Drugs 54:89–101
Schulz R, Guth BD, Pieper K, Martin C, Heusch G (1992) Recruitment of an inotropic reserve in moderately ischemic myocardium at the expense of metabolic recovery. Circ Res 70:1282–1295
Maekawa T, Yamamoto S, Igata Y, Ikeda S, Watanabe T, Shiraishi M (1997) Synthesis and biological activity of novel 2-(alpha-alkoxyimino) benzylpyridine derivatives as K+ channel openers. Chem Pharm Bull (Tokyo) 45:1994–2004
Teramoto N, Ito Y (1999) Comparative studies on the relaxing action of several adenosine 5′-triphosphate-sensitive K+ channel openers in pig urethra. J Smooth Muscle Res 35:11–22
Zhu BM, Miyamoto S, Nagasawa Y, Wajima T, Hashimoto K (2003) Effect of the sarcolemmal K(ATP) channel blocker HMR 1098 on arrhythmias induced by programmed electrical stimulation in canine old myocardial infarction model: comparison with glibenclamide. J Pharmacol Sci 93:106–113
Geelen P, O’Hara GE, Plante S, Philippon F, Gilbert M, Turgeon J (2000) Ischemia-induced action potential shortening is blunted by d-sotalol in a pig model of reversible myocardial ischemia. J Cardiovasc Pharmacol 35:638–645
Aupetit JF, Timour Q, Loufoua-Moundaga J et al (1995) Profibrillatory effects of lidocaine in the acute ischemic porcine heart. J Cardiovasc Pharmacol 25:810–816
Lygate CA, Hulbert K, Monfared M, Cole MA, Clarke K, Neubauer S (2003) The PPAR gamma-activator rosiglitazone does not alter remodeling but increases mortality in rats post-myocardial infarction. Cardiovasc Res 58:632–637
Pasnani JS, Ferrier GR (1992) Differential effects of glyburide on premature beats and ventricular tachycardia in an isolated tissue model of ischemia and reperfusion. J Pharm Exp Ther 262:1076–1084
Eto K, Ohya Y, Nakamura Y, Abe I, Fujishima M (2001) Comparative actions of insulin sensitizers on ion channels in vascular smooth muscle. Eur J Pharmacol 423:1–7
Ikeda S, Watanabe T (1998) Effects of troglitazone and pioglitazone on the action potentials and membrane currents of rabbit ventricular myocytes. Eur J Pharmacol 357:243–250
Knock GA, Mishra SK, Aaronson PI (1999) Differential effects of insulin-sensitizers troglitazone and rosiglitazone on ion currents in rat vascular myocytes. Eur J Pharmacol 368:103–109
Nakamura Y, Ohya Y, Onaka U, Fujii K, Abe I, Fujishima M (1998) Inhibitory action of insulin-sensitizing agents on calcium channels in smooth muscle cells from resistance arteries of guinea-pig. Br J Pharmacol 123:675–682
Billman GE, Englert HC, Schölkens BA (1998) HMR 1883, a novel cardioselective inhibitor of the ATP-sensitive potassium channel. Part II: effects on susceptibility to ventricular fibrillation induced by myocardial ischemia in conscious dogs. J Pharmacol Exp Ther 286:1465–1473
Wirth KJ, Klaus E, Englert HG, Schölkens BA, Linz W (1999) HMR 1883, a cardioselective K(ATP) channel blocker, inhibits ischaemia- and reperfusion-induced ventricular fibrillation in rats. Naunyn Schmiedebergs Arch Pharmacol 360:295–300
Dhein S, Pejman P, Krüsemann K (2000) Effects of the I(K.ATP) blockers glibenclamide and HMR1883 on cardiac electrophysiology during ischemia and reperfusion. Eur J Pharmacol 398:273–284
Zaugg M, Lucchinetti E, Spahn DR, Pasch T, Garcia C, Schaub MC (2002) Differential effects of anesthetics on mitochondrial KATP channel activity and cardiomyocyte protection. Anesthesiology 97:15–23
Kawano T, Oshita S, Takahashi A et al (2004) Molecular mechanisms of the inhibitory effects of propofol and thiamylal on sarcolemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology 100:338–346
Miller AK, DiCicco RA, Freed MI (2002) The effect of ranitidine on the pharmacokinetics of rosiglitazone in healthy adult male volunteers. Clin Ther 24:1062–1071
Kawai K, Kawasaki-Tokui Y, Odaka T et al (1997) Disposition and metabolism of the new oral antidiabetic drug troglitazone in rats, mice, and dogs. Arzneim-Forsch 47:356–368
Shigematsu S, Sato T, Abe T, Saikawa T, Sakata T, Arita M (1995) Persistent activation of ATP-sensitive K+ channels in early phase of reperfusion and its protective role against myocardial stunning. Circulation 92:2266–2275
Saavedra WF, Paolocci N, Kass DA (2002) Effects of cardioselective KATP channel antagonism on basal, stimulated, and ischaemic myocardial function in in vivo failing canine heart. Br J Pharmacol 135:657–662
Acknowledgements
The authors appreciate donations of crystalline troglitazone by Sankyo Pharmaceutical Research, Tokyo, Japan and of HMR-1098 by Sanofi-Aventis, Frankfurt, Germany, as well as assistance with computerised data analysis by J. Greyson. This study was supported by NIH grant HL49944 and the Medical Research Service of the Department of Veterans Affairs.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, L., Reiter, M.J., Xu, Y. et al. Thiazolidinedione drugs block cardiac KATP channels and may increase propensity for ischaemic ventricular fibrillation in pigs. Diabetologia 51, 675–685 (2008). https://doi.org/10.1007/s00125-008-0924-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-008-0924-0