Abstract
Aims/hypothesis
The aim of this study was to examine the incidence and trends of type 1 and type 2 diabetes in the 15–39 year-old population between 1992 and 1996 in Finland.
Subjects and methods
Data on the nationwide incidence of diabetes were obtained from four data sources: standardised reports from diabetes nurses, the Finnish National Hospital Discharge Register, the Drug Reimbursement Register and the Drug Prescription Register. The inclusion criterion was consistency in the diagnosis of diabetes across at least two data sources. The sex- and age-specific incidence was calculated for 5-year age groups, both for type 1 and type 2 diabetes. The effects of age, sex and year of diagnosis were assessed by fitting the linear regression model to the incidence data.
Results
Between 1992 and 1996 the age-adjusted incidence of type 1 diabetes among 15–39 year olds was 15.9 per 100,000/year. The incidence was highest among the 15–19 year olds and decreased with age. Conversely, the incidence of type 2 diabetes was very low among 15–19 year olds and increased with age. The total age-adjusted incidence of type 2 diabetes among 15–39 year olds was 11.8 per 100,000/year. The average annual increase in the incidence of type 2 diabetes was 7.9% (95% CI 3.7–12.2%).
Conclusions/interpretation
The age at which the Finnish population is at risk of type 1 diabetes extends into young adulthood. The rapid increase in the incidence of type 2 diabetes in the young adult population is a current public health problem.
Similar content being viewed by others
Introduction
Type 1 diabetes is defined by hyperglycaemia with an absolute deficiency in insulin secretion [1]. Individuals at increased risk of developing type 1 diabetes can often be identified by evidence of an immune-mediated process occurring in the pancreatic islets [2], which may be detected many years before disease onset. Type 2 diabetes is characterised by both resistance to the effect of insulin and the inability of beta cells to maintain an adequate level of insulin secretion [3]. Another specific type of diabetes includes diabetes caused by genetic defects affecting beta cell function or insulin action, other genetic syndromes associated with diabetes, diseases of exocrine pancreas, endocrinopathies, drug-induced or chemical-induced diabetes, or infection. Gestational diabetes represents a fourth type of this disease [1, 3].
The worldwide variation in the incidence of childhood type 1 diabetes within and between populations is remarkable [4, 5], and Finland has the highest incidence. A common feature of the incidence of type 1 diabetes in most populations is the increase in incidence with age up to puberty; however, in the Finnish population, a high incidence is seen even in pre-pubertal samples [6]. In most populations participating in the Diabetes Mondiale (DIAMOND) study, incidence increased with age and was found to be highest in children aged 10–14 years [5]. However, type 1 diabetes can develop at any age. It has been estimated that in approximately half of the cases the diagnosis of type 1 diabetes is made after the age of 15 years [7–9]. Recently published studies have shown that among young adults with type 1 diabetes, the proportion who received the diagnosis between the ages of 15 and 35 years varied from 76 to 91%, while the proportion of young adults with type 2 diabetes who received the diagnosis over the same age range varied from 7 to 24% [10–12].
The aetiology of type 1 diabetes is still largely unknown, but it is obvious that both genetic and environmental factors operate together to initiate a destructive process in the pancreatic beta cells, which leads to the onset of clinical diabetes. Reasons for the wide variability in the age of onset of type 1 diabetes are unclear. Furthermore, it has been suggested that the data on aetiological factors and natural history obtained in studies on diabetic children may not be fully applicable to type 1 diabetes patients diagnosed after childhood [13–17].
The incidence of type 1 diabetes among Finnish children younger than 15 years of age is the highest in the world [4, 5]; however, there are no data available on the incidence of diabetes incidence among young Finnish adults.
The clinical onset of type 2 diabetes typically occurs during adulthood, and the incidence increases with age [18, 19]. It has been assumed that type 2 diabetes is rare in individuals younger than 30 years of age, but in recent years there has been in increase in the incidence of type 2 diabetes in younger age groups [9, 20–23] and there is a paucity of population-based data on the proportion of young adults diagnosed with this disease [12].
The aim of the present register-based epidemiological study was to determine the incidence of diabetes in Finnish individuals aged 15–39 years.
Subjects and methods
Information on diagnoses of diabetes were collected on individuals aged 15–39 years who were diagnosed and resident in Finland between 1992 and 1996. The National Advisory Board on Health Care Ethics approved the study plan. Data were obtained from four different sources:
-
1.
All new cases of diabetes mellitus were reported to the Diabetes and Genetic Epidemiology Unit of the National Public Health Institute in Finland using standardised forms administered by diabetes nurses in hospitals and primary care diabetic clinics in Finland. The date of diagnosis of diabetes and the date of initiation of insulin treatment were included in the forms.
-
2.
The Drug Reimbursement Register of the Social Insurance Institute comprises information on persons entitled to free-of-charge medication for diabetes. Glucose-lowering agents (insulin and oral medication) prescribed by a physician are free of charge in Finland and are subject to the approval of a physician at the Institute who reviews each case history. Patients who apply for free-of-charge medication must attach a detailed medical statement prepared by the treating physician, who provides data to confirm the diagnosis of diabetes.
-
3.
Since late 1994, all prescriptions are included in the Drug Prescription Register of the Social Insurance Institute. Prescriptions searched for were for all the class A10 drugs in the WHO Anatomical Therapeutic Chemical Classification and Defined Daily Dose (drugs used in diabetes; insulin and analogues, blood glucose-lowering drugs, other drugs used in diabetes; available from http://www.whocc.no/atcddd/, last accessed in March 2007).
-
4.
The Finnish National Hospital Discharge Register maintained by the National Research and Development Centre for Welfare and Health includes up to four hospital discharge diagnoses for patients who have been admitted to the hospital ward. The treating physicians assign diagnostic codes using the International Classification of Diseases (ICD; ICD-9 until 1995 and ICD-10 from 1996 onwards; available from http://www.who.int/classifications/icd/en/, last accessed in March 2007). The Finnish versions of ICD-9 included also information about the type of diabetes.
The date of the first entry in one of these registers between 1992 and 1996 was used as the date of diagnosis. The diagnosis of diabetes was determined by linking the data from different registries using the unique personal identification number assigned to every Finnish resident in the 1970s and since then assigned at birth or immigration into the country. The inclusion criterion for the study was the consistent diagnosis of diabetes across at least two data sources. The type of diabetes was classified as type 1, type 2 or undefined type of diabetes using the information from the four data sources. The diagnoses made in hospitals were based on clinical characteristics, C-peptide measurements and, for a proportion of the patients, GAD antibody measurements. Detailed information on the medication taken was available from the Social Insurance Institute and the standardised forms were used in the classification as follows: (1) Only temporary entitlement to free-of-charge medication was counted as one source referring to type 2 diabetes, because patients with type 1 diabetes are given permanent entitlement at diagnosis. (2) Insulin administered immediately at diagnosis and continued until the end of the available information on the Drug Prescription Register (end of the year 2004) was considered to refer to type 1 diabetes. (3) Treatment with oral glucose-lowering agents alone was considered to refer to type 2 diabetes. (4) When insulin was first administered years after diagnosis conclusions could not be made as to the type of diabetes, as type 1 diabetes can have a slow onset in young adults, and insulin may be required during the natural course of type 2 diabetes.
Autoantibody measurements, apart from those used in the hospitals, were not used for classification as the study was register-based and blood was not drawn from the study subjects. The cases with equivocal information on the type of diabetes were investigated further. The original applications for free-of-charge medication were obtained from the Social Insurance Institute. The documents prepared by treating physicians were first reviewed by one of the authors (N. Lammi) for assignment of type of diabetes. The information available for the unclear cases who had not applied for free-of-charge medication were reviewed and classified by two professionals (N. Lammi and M. Karvonen). Most of these cases were found to have gestational diabetes and were excluded from the study. Patients who were categorised as having ‘other specific type of diabetes’ according to the American Diabetes Association definition (including genetic defects of the beta cell, genetic effects on insulin action, diseases of the exocrine pancreas, endocrinopathies, drug- and chemical-induced diabetes, infections, uncommon forms of immune-mediated diabetes and other genetic syndromes sometimes associated with diabetes) [3] were also excluded from the study.
A total of 5,195 cases were ascertained from the data sources (case found from at least one data source). The excluded cases were distributed as follows: there were 1,268 women diagnosed with gestational diabetes, 216 patients with secondary forms of diabetes, and 717 people whose diagnosis of diabetes could not be confirmed from two sources (of these 717 individuals, 16 could be found only in the Drug Reimbursement Register, 542 only in the Drug Prescription Register, 139 only in the Hospital Discharge Register and 20 only on the standardised form).
The completeness of registration was confirmed by estimating the degree of ascertainment using the capture–recapture method [24], where the standardised form was the primary source, and Hospital Discharge Register and Social Insurance Institution together formed the secondary source. The estimate of ascertainment for all diabetes cases (type 1, type 2 and undefined type together) was 88% (annual variation 88–90%).
Statistical methods
The sex- and age-specific incidence was calculated for 5-year age groups (15–19, 20–24, 25–29, 30–34 and 35–39 years). Age-standardised annual incidence rates were calculated using the WHO standard European population, where the age groups in question are identical in size. The observed cases were assumed to result from a Poisson distribution, and the exact 95% CIs were approximated as previously described [25]. The ratio of men:women was calculated for each age group and diabetes type, and the corresponding 95% CI was evaluated [26]. The effects of age, sex and year of diagnosis were assessed using a generalised linear model (glm) for Poisson family with a logarithmic link. Various covariate and cross-term combinations were considered, and the goodness-of-fit was compared using Akaike’s Criteria. All statistical analyses were performed using R software (available from http://www.R-project.org/, last accessed in March 2007).
Results
Of the 2994 diabetic patients aged 15–39 years when diagnosed with diabetes between 1992 and 1996 who were included in this analysis, 46.4% (1,388/2,994) had type 1 diabetes, 37.4% (1,121/2,994) had type 2 diabetes, and 16.2% (485/2,994) were classified as having undefined diabetes. The incidence rates according to sex and 5-year age group are shown in Table 1 and Fig. 1. In this section the incidence rates are presented per 100,000 of the population per year. The overall age-adjusted incidence of type 1 diabetes was 15.9. The incidence of type 1 diabetes was significantly higher in men than in women in all age groups. The overall ratio of men:women was 1.7 (incidence of 20.1 in men and 11.8 in women). For both sexes the incidence of type 1 diabetes was highest in the youngest age group (15–19 years) studied, in which it was 22.5 in total (27.1 in men and 17.6 in women), and decreased to 10.1 in the group aged 35–39 years (12.6 in men and 7.5 in women).
The overall incidence of type 2 diabetes among 15–39 year olds was 11.8 (12.7 in men and 11.0 in women). Contrary to the results for type 1 diabetes, the incidence of type 2 diabetes was low, with a rate of 0.5 in the youngest age group (0.4 in men and 0.6 in women), and increased with age, up to 29.9 among the 35–39 year olds (36.3 in men and 23.3 in women).
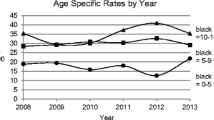
Statistical modelling indicated that age (p < 0.001) and sex (p < 0.001 for type 1 diabetes and p = 0.01 for type 2 diabetes) were both statistically significant factors that modify the incidence of both types of diabetes (Tables 2 and 3). Although there was no statistically significant trend in the incidence of type 1 diabetes over time, the age-standardised incidence was slightly increased among men and slightly decreased among women over the 5-year study period. The difference in incidence between sexes increased significantly with time at an average rate of 10.5% per year (Fig. 2a). The incidence of type 1 diabetes decreased with age at the same rate in both men and women, while the age profiles for the incidence of type 2 diabetes differed significantly between sexes (p < 0.001) (Table 3 and Fig. 2b). The incidence of type 2 diabetes increased, on average, by 7.9% (3.7–12.2%) per year between 1992 and 1996 (p < 0.001).
The incidence of undefined type of diabetes (including uncertain type 1 and type 2 diabetes and other unclassified forms of diabetes) was 5.3. The number of undefined cases of diabetes was smallest in the youngest age groups (9.2% in the group aged 15–19 years vs 17.5% group aged 35–39 years).
Discussion
This, the first nationwide study on the incidence of diabetes among 15–39 year olds in Finland, was based on linking the register data from several sources. The classification of diabetes was verified using the available data sources and, if necessary, complemented by reviewing records. The most important finding of the present study was probably the rapid annual increase (7.9%) in the incidence of type 2 diabetes among young adults. This increase was more than twofold greater than the previously reported annual increase in the incidence of type 1 diabetes in the population aged 14 years or younger (∼3%) [5, 6]. Among this young adult population, we did not observe an increase in the incidence of type 1 diabetes over the 5-year period of study. Although the incidence of type 1 diabetes decreased with age, the level in the group aged 35–39 years was still high. The incidence of type 1 diabetes among young adults is also relatively high in Finland compared with other countries [5]. Type 2 diabetes was rare among the youngest age group (15–19 years), but the incidence increased sharply with age. The rapidly increasing incidence of type 2 diabetes among young adults will be a notable public health problem in the near future, and if the incidence has continued to rise at the same rate since the study end, the problem may well already be apparent.
The national healthcare registers in Finland are reliable, and the coverage and the accuracy of them has been confirmed to be very good [27, 28]. In a previous study, the overall sensitivity of the Hospital Discharge Register and the Causes of Death Register for a diagnosis of myocardial infarction was 83% and the positive predictive value was 90% [27]. In addition, the Drug Reimbursement Register is a reliable data source because the reimbursement of medication costs provides the patient with a marked financial benefit; consequently, most drug-treated diabetic patients apply for this. Despite the good quality of Finnish register data, there are reasons to assume that the number of cases with type 2 diabetes was underestimated in this study. A Finnish prevalence study revealed that the proportion of undiagnosed type 2 diabetes patients was almost equal to the proportion of diagnosed patients among the population aged 45–64 years in 1992 [29]. Patients with type 2 diabetes who were treated with lifestyle counselling alone could not be captured using this method unless they were reported by diabetic nurses or were listed in the Hospital Discharge Register. Thus it can be assumed that some of the ‘milder’ cases of type 2 diabetes could not be captured. In addition, the Drug Prescription Register did not cover the first 2 years of the study period. Our estimations for the incidence rates reported here, particularly the incidence of type 2 diabetes, are therefore conservative.
Classification of diabetes in young adults is challenging and potential misclassification can happen, especially at the first encounter with a physician. In this study, 16.2% of the cases were classified as undefined, because some patients had discordant diagnoses of type 1 or type 2 diabetes using the different data sources. In rare cases, adequate information was not available for classification. However, the classification procedure used in this study ensures that all the cases in the ‘type-undefined group’ were diabetic. As the clinical onset of type 1 diabetes in adults may be less dramatic than in children, type 1 diabetes may masquerade as type 2 diabetes for several years [30, 31]. In particular, patients with atypical type 1 diabetes feature some of the characteristics of type 2 diabetes, such as the metabolic syndrome and a fluctuating need for insulin [31]. On the other hand, type 2 diabetes patients might present signs of systemic inflammation and might have autoantibodies against pancreatic beta cells [32–34]. HLA alleles and haplotypes associated with type 1 diabetes occur more frequently than expected in type 2 diabetic patients [34–36], and patients with type 2 diabetes have been reported to present with a decreased functional beta cell mass [35]. Taken together, it is obvious that the traditional classification of diabetes is very difficult, and it has been intensely criticised [37].
The incidence of type 1 diabetes in Finland is highest in the group aged 10–14 years, reaching a maximum during puberty (40.6 per 100,000/year) [38]. Among the young adult population of Finland the incidence of type 1 diabetes was highest in the youngest age group (15–19 years), but was considerably lower than in children aged younger than 15 years. A similar age distribution has been reported from other areas with a high incidence of childhood type 1 diabetes, such as Sweden, Sardinia (Italy) and Yorkshire (UK) [17]. It would appear that in the areas with a high incidence of childhood type 1 diabetes, the incidence falls steeply after 15 years of age, whereas in areas with a lower incidence of childhood type 1 diabetes, such as Catalonia (Spain) and Lithuania, the incidence decreases gradually with age or remains at the same level as observed in childhood [17]. However, despite the rapid fall in incidence after 15 years of age, the incidence of type 1 diabetes in young adults in Finland is still high compared with that in other countries [4, 5], and it seems that the age at which the Finnish population is at risk of type 1 diabetes extends into adulthood rather than being restricted to childhood or adolescence.
The higher incidence of type 1 diabetes in men compared with women among young adults is consistent with previous observations in Sweden [14], Italy [39] and Libya [40]. A slight male excess (ratio of men:women of 1.1) in the incidence of type 1 diabetes in children younger than 15 years in Finland has previously been reported [5]. The cause of the higher incidence in male children and adults is unknown. Possible explanations include external aetiological factors, such as increased susceptibility to viral infections [14], hormonal differences and the metabolic burden conferred by android fat distribution [17]. Moreover, the difference in type 1 diabetes incidence between sexes seems to be increasing in Finnish young adults.
The rising incidence of type 2 diabetes in young adults and adolescents has been globally acknowledged [21]. The incidence data are scarce, the highest numbers of type 2 diabetes in young adults have been reported in non-Europid ethnic groups [11, 21] but the age of diagnosis of type 2 diabetes has decreased also in Europid populations [41, 42]. Consistent with these reports, our results indicate that a considerable number of Finnish patients are diagnosed with type 2 diabetes before the age of 40. In Finland, the incidence of type 2 diabetes is high, but rates are difficult to compare because most previous studies on the occurrence of type 2 diabetes among young adults are based upon prevalence rates or have been case studies. However, it would appear that the incidence of type 2 diabetes in young adults in Finland does not reach the levels reported by studies conducted in the USA [43] or Libya [40].
The association between obesity and type 2 diabetes is well documented [44]. Concomitant with the rising incidence of type 2 diabetes, the proportion of overweight adolescents has been seen to continuously increase in Finland [45]. In recent reports, the prevalence of the metabolic syndrome has been shown to increase in parallel with increasing BMI and decreasing physical fitness in young Finnish men [46, 47]. However, the rate of increase in the incidence of type 2 diabetes in this study is considerably high, and extended surveillance of the incidence will show whether this trend is continuing. It is also noteworthy that the Drug Prescription Register did not cover the beginning of the study period, which might have caused some cases to fall out from the first examined years and consequently heighten the upward trend.
In conclusion, in addition to the burden conferred by the high incidence of type 1 diabetes in Finland, type 2 diabetes among the young adult population seems set to become an important public health problem in the near future. It has been shown that active lifestyle interventions are effective in the prevention of type 2 diabetes in the middle-aged adults [48], and these interventions should also be directed at younger populations.
Abbreviations
- ICD:
-
International Classification of Diseases
References
Genuth S, Alberti KG, Bennet P et al; The Expert Committee on the Diagnosis and Classification on Diabetes Mellitus (2003) Follow-up report on the diagnosis and classification of diabetes mellitus. Diabetes Care 26:3160–3167
Tuomilehto J, Zimmet P, Mackay IR et al (1994) Antibodies to glutamic acid decarboxylase as predictors of insulin-dependent diabetes mellitus before clinical onset of disease. Lancet 343:1383–1385
American Diabetes Association (2006) Diagnosis and classification of diabetes mellitus. Diabetes Care 29(Suppl 1):S43–S48
Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J (2000) Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 23:1516–1526
DIAMOND Project Group (2006) Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med 23:857–866
Karvonen M, Pitkäniemi J, Tuomilehto J; The Finnish Childhood Diabetes Registry Group (1999) The onset age of type 1 diabetes in Finnish Children has become younger. Diabetes Care 22:1066–1070
Melton LJ, Palumbo PJ, Chu CP (1983) Incidence of diabetes mellitus by clinical type. Diabetes Care 6:75–86
Lorenzen T, Pociot F, Hougaard P, Nerup J (1994) Long-term risk of IDDM in first-degree relatives of patients with IDDM. Diabetologia 37:321–327
Laakso M, Pyörälä K (1985) Age of onset and type of diabetes. Diabetes Care 8:114–117
Borg H, Arnqvist HJ, Bjork E et al (2003) Evaluation of the new ADA and WHO criteria for classification of diabetes mellitus in young adult people (15–34 yrs) in the Diabetes Incidence Study in Sweden (DISS). Diabetologia 46:173–181
Feltbower RG, McKinney PA, Campbell FM, Stephenson CR, Bodansky HJ (2003) Type 2 and other forms of diabetes in 0–30 year olds: a hospital based study in Leeds, UK. Arch Dis Child 88:676–679
Gungor N, Hannon T, Libman I, Bacha F, Arslanian S (2005) Type 2 diabetes mellitus in youth: the complete picture to date. Pediatr Clin North Am 52:1579–1609
Caillat-Zucman S, Garchon HJ, Timsit J et al (1992) Age-dependent HLA genetic heterogeneity of type 1 insulin-dependent diabetes mellitus. J Clin Invest 90:2242–2250
Blohme G, Nystrom L, Arnqvist HJ et al (1992) Male predominance of type 1 (insulin-dependent) diabetes mellitus in young adults: results from a 5-year prospective nationwide study of the 15–34-year age group in Sweden. Diabetologia 35:56–62
Vandewalle CL, Falorni A, Svanholm S, Lernmark A, Pipeleers DG, Gorus FK (1995) High diagnostic sensitivity of glutamate decarboxylase autoantibodies in insulin-dependent diabetes mellitus with clinical onset between age 20 and 40 years. The Belgian Diabetes Registry. J Clin Endocrinol Metab 80:846–851
Vandewalle CL, Falorni A, Lenrnmark A et al (1997) Associations of GAD65- and IA-2-autoantibodies with genetic risk markers in new-onset IDDM patients and their siblings. The Belgian Diabetes Registry. Diabetes Care 20:1547–1552
Kyvik KO, Nystrom L, Gorus F et al (2004) The epidemiology of type 1 diabetes mellitus is not the same in young adults as in children. Diabetologia 47:377–384
The DECODE Study Group (2003) Age and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care 26:61–69
The DECODA Study Group (2003) Age and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care 26:1770–1780
Libman I, Arslanian SA (1999) Type II diabetes mellitus: no longer just adults. Pediatr Ann 28:589–593
Rosenbloom AL, Joe JR, Young RS, Winter WE (1999) Emerging epidemic of type 2 diabetes in youth. Diabetes Care 22:345–354
Fagot-Campagna A, Pettitt DJ, Engelgau MM et al (2000) Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 136:664–672
Owen KR, Stride A, Ellard S, Hattersley AT (2003) Etiological investigation of diabetes in young adults presenting with apparent type 2 diabetes. Diabetes Care 26:2088–2093
LaPorte RE, McCarty D, Bruno C, Tajima N, Baba S (1993) Counting diabetes in the next millennium. Application of capture-recapture technology. Diabetes Care 16:528–534
Anderson RN, Rosenberg HM (1998) Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep 47:1–16, 20
Rothman KJ, Greenland S (1998) Modern epidemiology, 2nd edn. Lippincott-Raven, Philadelphia, pp 237–239
Pajunen P, Koukkunen H, Ketonen M et al (2005) The validity of the Finnish hospital discharge register and causes of death register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil 12:132–137
Mahonen M, Miettinen H, Pyorälä K, Molarius A, Salomaa V, Kuulasmaa K (1995) Hospital discharge register data in the assessment of trends in acute myocardial infarction. FINMONICA AMI Register Study Team. Ann Med 27:547–554
Ylihärsilä H, Lindström J, Eriksson JG et al (2005) Prevalence of diabetes and impaired glucose regulation in 45- to 64-year-old individuals in three areas of Finland. Diabet Med 22:88–91
Bushhouse SA, Goetz FC, Jacobs DR Jr et al (1992) C-peptide response to meal challenge in nondiabetic and diabetic adults living in Wadena, Minnesota. Diabetes Care 15:1335–1347
Aguilera E, Casamitjana R, Ercilla G, Oriola J, Gomis R, Conget I (2004) Adult-onset atypical (type 1) diabetes: additional insights and differences with type 1A diabetes in a European Mediterranean population. Diabetes Care 27:1108–1114
Pietropaolo M, Barinas-Mitchell E, Pietropaolo SL, Kuller LH, Trucco M (2000) Evidence of islet cell autoimmunity in elderly patients with type 2 diabetes. Diabetes 49:32–38
Kolb H, Mandrup-Poulsen T (2005) An immune origin of type 2 diabetes? Diabetologia 48:1038–1050
Gilliam LK, Brooks-Worrell BM, Palmer JP, Greenbaum CJ, Pihoker C (2005) Autoimmunity and clinical course in children with type 1, type 2, and type 1.5 diabetes. J Autoimmun 25:244–250
Donath MY, Ehses JA (2006) Type 1, type 1.5, and type 2 diabetes: NOD the diabetes we thought it was. Proc Natl Acad Sci USA 103:12217–12218
Tuomilehto-Wolf E, Tuomilehto J, Hitman GA et al (1993). Genetic susceptibility to non-insulin dependent diabetes mellitus and glucose intolerance are located in HLA-region. BMJ 307:155–159
Gale EA (2006) Declassifying diabetes. Diabetologia 49:1989–1995
Rytkönen M (2004) Geographical study on childhood type 1 diabetes mellitus (T1DM) in Finland. Oulu University Press, Oulu
Bruno G, Cerutti F, Merletti F et al (2005) Residual beta-cell function and male/female ratio are higher in incident young adults than in children: the registry of type 1 diabetes of the province of Turin, Italy, 1984–2000. Diabetes Care 28:312–317
Kadiki OA, Reddy MR, Marzouk AA (1996) Incidence of insulin-dependent diabetes (IDDM) and non-insulin-dependent diabetes (NIDDM) (0–34 years at onset) in Benghazi, Libya. Diabetes Res Clin Pract 32:165–173
Wiegand S, Maikowski U, Blankenstein O, Biebermann H, Tarnow P, Gruters A (2004) Type 2 diabetes and impaired glucose tolerance in European children and adolescents with obesity—a problem that is no longer restricted to minority groups. Eur J Endocrinol 151:199–206
Koopman RJ, Mainous AG, Diaz VA, Geesey ME (2005) Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 3:60–63
SEARCH for Diabetes in Youth Study Group (2006) The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for diabetes in youth study. Pediatrics 118:1510–1518
Smyth S, Heron A (2006) Diabetes and obesity: the twin epidemics. Nat Med 12:75–80
Kautiainen S, Rimpelä A, Vikat A, Virtanen SM (2002) Secular trends in overweight and obesity among Finnish adolescents in 1977–1999. Int J Obes Relat Metab Disord 26:544–552
Mikkola I, Keinänen-Kiukaanniemi S, Laakso M et al (2007) Metabolic syndrome in connection with BMI in young Finnish male adults. Diabetes Res Clin Pract 76:404–409
Santtila M, Kyröläinen H, Vasankari T et al (2006) Physical fitness profiles in young Finnish men during the years 1975–2004. Med Sci Sports Exerc 38:1990–1994
Lindstrom J, Ilanne-Parikka P, Peltonen M et al (2006) Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 368:1673–1679
Acknowledgements
This work was funded by the NIH-grant No: DK062374-01A1 and the grants of the Academy of Finland No: 207008 and No: 21411.
Duality of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lammi, N., Taskinen, O., Moltchanova, E. et al. A high incidence of type 1 diabetes and an alarming increase in the incidence of type 2 diabetes among young adults in Finland between 1992 and 1996. Diabetologia 50, 1393–1400 (2007). https://doi.org/10.1007/s00125-007-0690-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0690-4