Abstract
Aims/hypothesis
Atorvastatin exerts beneficial vascular effects in diabetes, but the underlying mechanisms are yet to be elucidated. The aim of the present study was to determine whether Rac-1 is involved in the effect of atorvastatin on oxidative stress and vascular dysfunction.
Materials and methods
Using human aortic endothelial cells (HAECs) we evaluated the effect of high glucose levels on peroxide production by dihydrodichlorofluorescein and on Rac-1 activity using immunocytochemistry to detect Rac-1 translocation to the membrane. We evaluated vascular function, peroxide production by dihydroethidium and NADPH oxidase activity in vessels from atorvastatin-treated mice. Rac-1 activity was also assessed, both by immunoprecipitation of the Rac–p21-activated kinase complex and by analysis of Rac-1 translocation to the membrane. These experiments were also conducted in vessels infected with an adenoviral vector carrying a constitutively active mutant of Rac-1.
Results
In HAECs exposed to high glucose levels, atorvastatin prevented oxidative stress, and this protection was associated with impaired Rac-1 activation. This effect was also observed in a murine model of diabetes mellitus. More importantly, the addition of geranylgeranyl pyrophosphate (GGPP) blocked the effects of atorvastatin in both glucose-exposed HAECs and diabetic vessels. Atorvastatin failed to afford protection against vascular abnormalities in the presence of a constitutively active mutant of Rac-1.
Conclusions/interpretation
The results of this study demonstrate that the vascular antioxidant effect of atorvastatin in diabetes is mediated through inhibition of Rac-1 via a reduction in GGPP. Thus, selective Rac-1 inhibition should be considered in the design of novel pharmacological strategies to reduce the impact of diabetes mellitus on vascular function.
Similar content being viewed by others
Introduction
Diabetes mellitus is increasing at epidemic proportions throughout the world and is considered one of the main threats to human health. The injury induced by diabetes mellitus on micro- and macrovessels is considered the main causes of increased morbidity and mortality in this disease, and over the last decade novel treatments have been adopted to reduce the negative effect of diabetes mellitus on vascular function [1–3]. It has been reported that statins (3-hydroxy-3-methylglutaryl CoA reductase inhibitors)—a class of drugs mainly characterised by their cholesterol-lowering effects—exert beneficial vascular effects even in diabetes mellitus [4–6]. In particular, treatment with statins protects against micro- and macrovascular complications and reduces the cardiovascular risk observed in diabetic patients [7–9]. These beneficial vascular effects have also been observed in diabetic patients with normal cholesterol levels, suggesting that the clinical benefits of statins may be independent of their cholesterol-lowering effects and could be the result of a direct action on the vascular wall [10, 11]. It has been demonstrated that acute treatment with statins rescues endothelial dysfunction induced by diabetes mellitus, and this favourable effect has been ascribed to restriction of the oxygen free radical production induced by hyperglycaemia [12]. However, the molecular mechanisms underlying these vascular effects of statins have been not completely elucidated.
Through inhibition of l-mevalonic acid production, statins prevent the synthesis not only of cholesterol, but also of important isoprenoid intermediates of the cholesterol biosynthetic pathway, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which are required for the lipid-modification of proteins involved in intracellular signal transduction. Prenylation with farnesyl or the geranylgeranyl group of small GTP-binding proteins, such as members of Ras and Rho GTPase families, is needed for translocation to the plasma membrane [13, 14]. Therefore, post-translational modification of small GTP-binding proteins by isoprenylation could be another important target of statin treatment.
Among the members of the Rho family, we have recently demonstrated that Rac-1 is involved in the vascular oxidative stress induced by high glucose levels [15]. It is therefore reasonable to hypothesise that the beneficial effect on vascular function induced by statins in diabetes mellitus is due to inhibition of Rac-1 activity through a reduction in the availability of isoprenoid intermediates. The aim of the present study was to test this hypothesis by evaluating the effect of atorvastatin on Rac-1 trafficking in human aortic endothelial cells (HAECs) in the presence of high glucose levels and in a mouse model of diabetes mellitus.
Materials and methods
Cell culture
HAECs were obtained from Clonetics (Cambrex, East Rutherford, NJ, USA). Cells were cultured in endothelial growth medium as previously described [15].
Evaluation of superoxide production in endothelial cells
HAECs were cultured, serum deprived, and then incubated with or without 10 μmol/l atorvastatin for 24 h. Cells were treated with high (30 mmol/l) glucose levels for 6 h, either alone or in combination with FPP (10 μmol/l Sigma-Aldrich, St Louis, MO, USA) or GGPP (10 μmol/l Sigma-Aldrich). Cells were subsequently loaded with 10 μmol/l dihydrodichlorofluorescein (DCHF) diacetate (Sigma-Aldrich) for 15 min, and observed under a microscope [15]. Twenty-four-bit colour pictures were acquired and analysed using a digital camera system coupled to imaging software (Spot, Diagnostic Instruments, Sterling Heights, MI, USA) under constant exposure time, gain and offset, chosen so as to increase the threshold for fluorescence. Peroxide production was evaluated by selectively measuring green fluorescence intensity corrected for background fluorescence and expressed as arbitrary units. All images were taken in the first 30 s of light exposure, during which time no fluorescence decay was detected in preliminary studies.
Intracellular Rac-1 translocation
HAECs were seeded in chamber slides, grown to confluence, and then serum deprived. Cells were treated as previously described. At the end of treatments, cells were fixed with 4% paraformaldehyde. After blocking with 1% BSA, permeabilisation was obtained with 0.01% Triton X-100, and cells were incubated with anti-Rac-1 primary antibody (1:250; BD Bioscience, Canada) and anti-rabbit Cy3 rhodamine secondary antibodies [15]. Images were acquired and fluorescence intensity was measured as described above.
Animals
C57BL/6 mice (Charles River, Calco, Italy) were kept in standard cages under a 12-h light–dark cycle and had free access to food. Mice were injected with streptozotocin (200 mg/kg i.p.; n = 18) or vehicle (control; n = 18) [15–17]. After 14 days, blood glucose levels were measured to confirm induction of diabetes mellitus. The animals were then killed, and aorta, carotid and mesenteric arteries were excised and cleaned of periadventitial tissue for subsequent experiments. A further group of animals (n = 20) was treated with 1 mg kg−1 day−1 of atorvastatin or vehicle (PBS) for 9 days before the induction of diabetes mellitus, as described above. All studies were performed according to our institutional guidelines.
Evaluation of vascular reactivity
Vascular reactivity studies were performed on aortas as previously described [15, 18, 19]. Vasorelaxation in response to acetylcholine (10−9−10−5 mol/l; Sigma-Aldrich) and sodium nitroprusside (10−10−10−6 mol/l; Sigma-Aldrich) was expressed as the percentage reduction in contraction obtained with phenylephrine (10−6 mol/l; Sigma-Aldrich). Some of the vessels from atorvastatin-treated mice were incubated with GGPP (10 μmol/l) or FPP (10 μmol/l) for 15 min before performing the study.
Measurement of ex vivo aortic superoxide production
We have previously shown that the diabetes-induced increase in lucigenin-enhanced chemiluminescence is blocked using the NADPH oxidase inhibitor apocynin, suggesting that the increase is due to superoxide production by NADPH oxidase [15].
Segments of thoracic aorta were isolated, placed in a modified Krebs buffer containing 20 mmol/l sodium HEPES, and allowed to equilibrate for 30 min at 37°C. After 5 min of dark adaptation, scintillation vials containing 2 ml of Krebs-HEPES buffer with 5 μmol/l lucigenin were placed into a scintillation counter switched to the out-of-coincidence mode. Chemiluminescence values were obtained at 40-s intervals over 15 min, and readings were averaged. The vessel was subsequently dried and the dry weight was measured. Lucigenin counts were expressed as cpm/mg dry weight of the vessel. Background counts were determined from vessel-free incubations and subtracted from vessel readings.
Evaluation of superoxide production in vessels
Superoxide production was assessed by dihydroethidium (DHE) dyeing, as previously described [15, 19]. DHE emits a red fluorescent signal after binding to these oxygen free radicals and therefore directly reflects superoxide levels. After excision, vessels were placed in Killik medium (Biooptica, Milan, Italy) and frozen. Consecutive sections (20 μm thick) were cut in a Jung CM3000 cryostat (Leica Microsystems, Wetzlar, Germany) and placed on microscope slides. Sections were incubated with DHE (10 μmol/l; Sigma-Aldrich) in Krebs buffer for 30 min, during which time they were protected from light. Images were acquired and fluorescence intensity was measured as described above.
NADPH oxidase enzymatic activity
Although the activation of several different enzymes can result in oxidative stress, it is well known that reactive oxygen species (ROS) in vessels are mainly derived from NADPH oxidase enzymatic activity. We therefore evaluated NADPH oxidase enzymatic activity in vascular tissues from diabetic mice and relative control mice which either did or did not receive atorvastatin treatement. NADPH oxidase activity was measured using a lucigenin assay, as previously described [15, 19]. Vessels were lysed in a buffer containing protease inhibitors (20 mmol/l monobasic potassium phosphate, 1 mmol/l EGTA, 10 μmol/l aprotinin, leupeptin, pepstatin, 0.5 mmol/l phenylmethylsulfonyl fluoride, pH 7.0). Lysate protein was collected and resuspended in TRIS-sucrose buffer, and then protein content was measured by the Bradford method. NADPH oxidase activity was performed in a phosphate buffer (50 mmol/l, pH 7.0) containing 1 mmol/l EGTA, 150 mmol/l sucrose, 5 μmol/l lucigenin as the electron acceptor, and NADPH (100 μmol/l) as the substrate (final volume 2 ml). The reaction was initiated by the addition of 100 μg of protein. Photon emission was measured every 30 s for 30 min in a scintillation counter. Background counts were determined by protein-free incubations and were subtracted from protein readings. Results were expressed in cpm mg protein−1 min−1.
Rac-1 activity assay
The activity of Rac-1 can be monitored by its interaction with p21-activated kinase (PAK), which only occurs when Rac-1 is active. Vessels were excised and incubated in Krebs buffer. They were then lysed in a buffer containing NP-40. Lysates stimulated with GTPγS were used as a positive control. A PAK-glutathione S-transferase (GST) fusion protein bound to agarose beads was added, and active Rac-1 bound to PAK-GST was separated from the beads by repetitive centrifugation and washing. The samples were subsequently separated by SDS-PAGE and transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). The membrane was immunoblotted with anti-Rac-1 antibody (Upstate, Charlottesville, VA, USA). Rac-1 levels were quantified by western blotting [15, 19]. Rac-1 translocation to the plasma membrane in vascular tissues was evaluated as previously described [15].
Adenoviral infection of carotid artery
Carotid arteries from diabetic mice treated or not with atorvastatin were infected with adenoviral vectors, as previously described [15, 19]. Carotid arteries were placed in a Mulvany pressure system (Danish Myo Technology, Aarhus, Denmark) with DMEM/F12 medium, containing 250 μl of AdV12 (vector containing a constitutively active Rac-1) or Ad0 (empty vector) at a concentration of 1 × 109 pfu/ml. Vessels were perfused at a pressure of 100 mmHg for 1 h and then 60 mmHg for 5 h. The efficiency of infection was >80% in all vascular tissues, as indicated by levels of green fluorescent protein (GFP), which was co-expressed. AdV12 and Ad0 were gifts from E. Hirsh and G. Tarone (University of Turin, Italy).
Statistical analysis
Data are presented as means±SEM. Statistical analysis was performed by two-way ANOVA followed by the Bonferroni post hoc test. Differences were considered to be statistically significant at p < 0.05.
Results
Atorvastatin reduces Rac-1 translocation and oxidative stress in endothelial cells exposed to high glucose
In HAECs, high glucose induced the translocation of Rac-1 to the plasma membrane (Fig. 1a–d) and increased DHCF fluorescence (Fig. 1e–i), a marker of ROS production. Interestingly, atorvastatin treatment prevented both effects. The addition of GGPP, but not FPP, abolished the effects of atorvastatin on high glucose-induced Rac-1 translocation and DHCF fluorescence, but had no effect under control conditions (data not shown).
Representative micrographs showing Rac-1 localisation (a–d) and DCHF (e–h) in HAECs under control conditions (a) or in the presence of high glucose levels (b), high glucose plus atorvastatin (c) or high glucose plus atorvastatin and GGPP (d). Arrows indicate Rac-1 immunopositivity. i Quantification of DCHF fluorescence. **p < 0.01 vs control (n = 5)
Atorvastatin blunts vascular Rac-1 activity and oxidative stress in diabetes mellitus
Mice treated with streptozotocin had higher plasma glucose levels (25.3 ± 1.0 mmol/l; n = 18) than vehicle-treated mice (5.0 ± 0.2 mmol/l; n = 18). The hyperglycaemia induced by streptozotocin was unaffected by atorvastatin treatment (25.6 ± 1.0 mmol/l; n = 20).
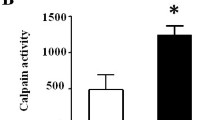
Diabetic mice displayed impaired acetylcholine-induced vasorelaxation (Fig. 2a), and this was associated with increased oxygen free radical production (Fig. 2b–e) and NADPH oxidase activity (Fig. 3a). Concomitantly, increased vascular Rac-1 activity, evaluated both as Rac-1–PAK complex and Rac-1 translocation from the cytoplasm to the plasma membrane was observed in diabetic mice (Fig. 3b,c). All these hyperglycaemia-induced vascular abnormalities were rescued by the administration of atorvastatin (Figs. 2 and 3). Interestingly, the addition of GGPP (Fig. 2a), but not FPP (data not shown), blunted the improvement in vascular function induced by atorvastatin.
a Vascular response to increasing doses of acetylcholine on aortic rings in control mice (filled circles) or diabetic mice not treated (filled triangle) or treated with atorvastatin in the presence (empty triangle) or absence (empty circle) of GGPP. (**p < 0.01 vs control; n = 7). b Lucigenin-enhanced chemiluminescence in aortic rings of control mice (Control) or diabetic mice without or with atorvastatin (**p < 0.01 vs control; n = 5). DHE fluorescence in carotid of control mice (c) and diabetic mice without (d) or with (e) atorvastatin (n = 5)
a Vascular NADPH oxidase enzymatic activity in control mice, untreated diabetic mice, and diabetic mice treated with atorvastatin (**p < 0.01 vs control; n = 5). b Representative western blots (n = 4) of the vascular Rac-1–PAK complex in control mice, diabetic mice and diabetic mice treated with atorvastatin. c Representative western blots (n = 4) of vascular Rac-1 in the membrane (mem) and cytoplasm (cyt) in control mice, diabetic mice and diabetic mice treated with atorvastatin. IP immunoprecipitate, WB western blotting
The vascular response to nitroglycerine was not affected by either diabetes or atorvastatin treatment (data not shown).
Constitutively active Rac-1 (AdV12) abolishes the beneficial vascular effects produced by atorvastatin treatment
In vessels from diabetic mice treated with atorvastatin, Rac-1 activity was higher in those infected with AdV12 than in those treated with Ad0. This suggests that atorvastatin treatment is unable to blunt Rac-1 activity in diabetic vessels in the presence of AdV12 (Fig. 4a). More importantly, under these conditions, atorvastatin treatment failed to prevent diabetes-induced vascular oxidative stress (Fig. 4b) and, consequently, to improve acetylcholine-induced vasorelaxation (Fig. 4c).
a Representative western blots (n = 4) of the vascular Rac-1–PAK complex in untreated diabetic mice, and diabetic mice treated with atorvastatin and infected with AdV12 or Ad0. b Lucigenin chemiluminescence in aortic rings of untreated diabetic mice, and diabetic mice treated with atorvastatin and infected with AdV12 or Ad0 (**p < 0.01 vs control; n = 4). The insert shows micrographs of GFP green fluorescence in control (left-hand insert) and AdV12-infected (right-hand insert) infected carotid arteries (n = 3). c Vascular response to increasing doses of acetylcholine in aortic rings in untreated diabetic mice (filled triangles) and diabetic mice treated with atorvastatin and infected with Ad0 (empty circles) or AdV12 (filled circles). (**p < 0.01 vs control; n = 5). IP immunoprecipitate, WB western blotting
Vascular relaxation induced by nitroglycerine was unaffected by either AdV12 or Ad0 (data not shown).
Discussion
The results of the present study add to our knowledge on the molecular mechanisms that mediate the beneficial effects of statins under pathological conditions characterised by increased vascular oxidative stress, such as diabetes mellitus. Specifically, our results demonstrate that the protective antioxidant effect of atorvastatin on vascular function in diabetes mellitus is mediated by the inhibition of Rac-1 activity. This conclusion is based on results, obtained under several experimental conditions, showing that the effect of atorvastatin on diabetes-induced oxidative stress is regularly accompanied by a blunted Rac-1 activity. More importantly, in the presence of a constitutively active Rac-1 mutant, atorvastatin failed to exert its protective vascular effects.
Several mechanisms have been postulated to be responsible for the beneficial action of statins on endothelium, such as the overproduction of endothelial nitric oxide synthase [20, 21], a reduction in endothelin-1 synthesis [22], and the inhibition of ROS production [20, 23]. In diabetes mellitus, however, the main contributor to vascular injury is an excess of ROS, which is produced as a result of long-standing hyperglycaemic stress [15, 24–26]. Therefore, in the pathogenesis of diabetes-induced endothelial dysfunction, the beneficial actions of statins are related to the reduction of oxidative stress [25] and are independent of its effect on cholesterol synthesis. This is fully supported by our studies on isolated endothelial cells and vessels, which revealed that the addition of FPP, an intermediate that rescues cholesterol synthesis, does not influence the effects of atorvastatin on oxidative stress or vasorelaxation. In contrast, the addition of GGPP, a product of two intermediate metabolites of cholesterol synthesis that is unable per se to rescue cholesterol synthesis, prevents the effects of atorvastatin.
The results of the present study provide further evidence that the antioxidant effect of statins in diabetes mellitus reflects the ability of these agents to modulate small GTPases such as Rac-1, an important molecular switch. Rac-1 integrates diverse stimuli in the cardiovascular system at an intracellular level and plays a role in key signalling functions such as those involved in superoxide production [27, 28]. This protein is a regulatory component of NADPH oxidase, which is one of the major sources of oxidative stress in the vascular wall [15, 27, 29]. Isoprenylation of Rac-1 with GGPP is necessary to anchor it to the plasma membrane, where it triggers the clustering of the subunits of NADPH oxidase to form the functional multienzyme complex, which, in turn, leads to ROS production [28].
In the present paper we show that Rac-1 translocation from the cytosol to the plasma membrane is inhibited by atorvastatin, resulting in reduced hyperglycaemia-driven ROS production. Moreover, our results indicate that the action of atorvastatin on Rac-1 is dependent on the depletion of intracellular stores of GGPP since, in the presence of atorvastatin, exogenous administration of GGPP restores hyperglycaemia-induced Rac-1 translocation and oxidative stress. These data are in agreement with our previous observations demonstrating that withdrawal of atorvastatin from endothelial cells induces Rac-1 translocation to plasma membrane and, consequently, oxidative stress [29].
Our data in diabetic mice provide further evidence of the importance of Rac-1 inhibition in the vascular antioxidant effect of statins. Of note, the administration of GGPP eliminates the beneficial effects of atorvastatin on vascular function, indicating that the reduction in isoprenoid metabolites and consequent inhibition of isoprenylation is the key event responsible for the improvement in vascular function observed during atorvastatin treatment. A reduction in the isoprenylation of Rac-1 is therefore a possible explanation for the blunted production of ROS by NADPH oxidase during atorvastatin treatment.
We performed further experiments using a genetic approach. Specifically, we circumvented the inhibition of Rac-1 activation by infecting diabetic vessels with a constitutively active Rac-1 mutant. Under these experimental conditions we observed that Rac-1 interacts with PAK even in presence of atorvastatin. Moreover, in presence of the constitutively active Rac-1 mutant, atorvastatin failed to counteract oxidative stress and improve vascular function. These data clearly indicate that Rac-1 is key mediator of the protective functions of statins in diabetes mellitus and that the other molecular mechanisms stimulated by atorvastatin play only an accessory role. Furthermore, our experimental evidence further supports the concept that restriction of vascular oxidative stress is a fundamental goal in the treatment of diabetes mellitus.
In conclusion, our study demonstrates that inhibition of Rac-1 is crucial for the beneficial effects exerted by statins on endothelial function in diabetes mellitus. This suggests that selective Rac-1 inhibition could be exploited in the search for more precisely targeted pharmacological approaches to reduce cardiovascular risk in diabetic patients.
Abbreviations
- DCHF:
-
dihydrodichlorofluorescein
- DHE:
-
dihydroethidium
- FPP:
-
farnesyl pyrophosphate
- GFP:
-
green fluorescent protein
- GGPP:
-
geranylgeranyl pyrophosphate
- GST:
-
glutathione S-transferase
- HAECs:
-
human aortic endothelial cells
- PAK:
-
p21-activated kinase
- Phe:
-
phenylephrine
- ROS:
-
reactive oxygen species
- SNP:
-
sodium nitroprusside
References
King GL, Buzney SM, Kahn CR et al (1983) Differential responsiveness to insulin of endothelial and support cells from micro- and macrovessels. J Clin Invest 71:974–979
Laakso M (1999) Hyperglycemia and cardiovascular disease in type II diabetes. Diabetes 48:937–942
Meigs JB, O’Donnell CJ, Tofler GH et al (2006) Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes 55:530–537
Colhoun HM, Betteridge DJ, Durrington PN et al (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364:685–696
Collins R, Armitage J, Parish S, Sleight P, Peto R; Heart Protection Study Collaborative Group (2004) Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high-risk conditions. Lancet 363:757–767
Collins R, Armitage J, Parish S, Sleigh P, Peto R (2003) Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 361:2005–2016
Sakamoto K, Murata T, Chuma H, Hori M, Ozaki H (2005) Fluvastatin prevents vascular hyperplasia by inhibiting phenotype modulation and proliferation through extracellular signal-regulated kinase 1 and 2 and p38 mitogen-activated protein kinase inactivation in organ-cultured artery. Arterioscler Thromb Vasc Biol 25:327–333
Shinohara K, Shoji T, Kimoto E et al (2005) Effect of atorvastatin on regional arterial stiffness in patients with type 2 diabetes mellitus. J Atheroscler Thromb 12:205–210
Mooradian AD, Haas MJ, Batejko O, Hovsepyan M, Feman SS (2005) Statins ameliorate endothelial barrier permeability changes in the cerebral tissue of streptozotocin-induced diabetic rats. Diabetes 54:2977–2982
Mihaylova B, Briggs A, Armitage J, Parish S, Gray A, Collins R; Heart Protection Study Collaborative Group (2005) Cost-effectiveness of simvastatin in people at different levels of vascular disease risk: economic analysis of a randomised trial in 20,536 individuals. Lancet 365:1779–1785
Beckman JA, Liao JK, Hurley S et al (2004) Atorvastatin restores endothelial function in normocholesterolemic smokers independent of changes in low-density lipoprotein. Circ Res 95:217–223
Christ M, Bauersachs J, Liebetrau C, Heck M, Gunther A, Wehling M (2002) Glucose increases endothelial-dependent superoxide formation in coronary arteries by NADPH oxidase activation: attenuation by the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor atorvastatin. Diabetes 51:2648–2652
Kinsella BT, Erdman RA, Maltese WA (1991) Carboxyl-terminal isoprenylation of ras-related GTP binding proteins encoded by rac1, rac2 and ralA. J Biol Chem 266:9786–9794
Gorzalczany Y, Sigal N, Itan M, Lotan O, Pick E (2000) Targeting of Rac1 to the phagocyte membrane is sufficient for the induction of NADPH oxidase assembly. J Biol Chem 275:40073–40081
Vecchione C, Aretini A, Marino G et al (2006) Selective Rac-1 inhibition protects from diabetes-induced vascular injury. Circ Res 98:218–225
Burkart V, Wang ZQ, Radons J et al (1999) Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic beta-cell destruction and diabetes development induced by streptozotocin. Nat Med 5:314–319
Soriano FG, Virag L, Jagtap P et al (2001) Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med 7:108–113
Vecchione C, Fratta L, Rizzoni D et al (2002) Cardiovascular influences of alpha1b-adrenergic receptor defect in mice. Circulation 105:1700–1707
Vecchione C, Patrucco E, Marino G et al (2005) Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3K. J Exp Med 201:1217–1228
Endres M, Laufs U (2004) Effects of statins on endothelium and signalling mechanisms. Stroke 35:2708–2711
Harris MB, Blackstone MA, Sood SG et al (2004) Acute activation and phosphorylation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitors. Am J Physiol Heart Circ Physiol 287:H560–H566
Mraiche F, Cena J, Das D, Vollrath B (2005) Effects of statins on vascular function of endothelin-1. Br J Pharmacol 144:715–726
Maack C, Kartes T, Kilter H et al (2003) Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 108:1567–1574
Baynes JW (1991) Role of oxidative stress in development of complications in diabetes. Diabetes 40:405–412
Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA (1996) Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 97:22–28
Naka Y, Bucciarelli LG, Wendt T et al (2004) RAGE axis: Animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol 24:1342–1349
Diekmann D, Abo A, Johnston C, Segal AW, Hall A (1994) Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science 265:531–533
Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW (1991) Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353:668–670
Vecchione C, Brandes RP (2002) Withdrawal of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors elicits oxidative stress and induces endothelial dysfunction in mice. Circ Res 91:173–179
Acknowledgements
This work was partly supported by Italian Ministry of Research (Ricerca Corrente).
Duality of interest
We state that we do not have any duality of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vecchione, C., Gentile, M.T., Aretini, A. et al. A novel mechanism of action for statins against diabetes-induced oxidative stress. Diabetologia 50, 874–880 (2007). https://doi.org/10.1007/s00125-007-0597-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0597-0