Abstract
Aims/hypothesis
Hyperlipidaemia often occurs in patients with type 2 diabetes mellitus. Though HMG-CoA reductase inhibitors (statins) are widely used for controlling hypercholesterolemia, atorvastatin has also been reported to have an adverse effect on glucose metabolism. Based on these findings, the aim of this study was to investigate the effects of statins on adipocytes, which play pivotal roles in glucose metabolism.
Methods
In 3T3-L1 cells, effects of statins on adipocyte maturation were determined morphologically. Protein and mRNA levels of SLC2A4 and adipocyte marker proteins were determined by immunoblotting and RT-PCR, respectively. Type 2 diabetic NSY mice were treated with atorvastatin for 15 weeks, followed by glucose and insulin tolerance tests and examination of SLC2A4 expression in white adipose tissue (WAT). Seventy-eight Japanese subjects with type 2 diabetes and hypercholesterolaemia were treated with atorvastatin (10 mg/day), and its effects on lipid and glycaemic profiles were measured 12 weeks after treatment initiation.
Results
Treatment with atorvastatin inhibited adipocyte maturation, SLC2A4 and C/EBPα expressions and insulin action in 3T3-L1 cells. Atorvastatin also attenuated SLC2A4 and C/EBPα expressions in differentiated 3T3-L1 adipocytes. These effects were reversed by l-mevalonate or geranylgeranyl pyrophosphate. In NSY mice, atorvastatin accelerated glucose intolerance as a result of insulin resistance and decreased SLC2A4 expression in WAT. In addition to improving hyperlipidaemia, atorvastatin treatment significantly increased HbA1c but not fasting glucose levels in diabetic patients, and this effect was greater in the non-obese subgroup.
Conclusions/interpretation
These results demonstrate that atorvastatin attenuates adipocyte maturation and SLC2A4 expression by inhibiting isoprenoid biosynthesis, and impairs glucose tolerance. These actions of atorvastatin could potentially affect the control of type 2 diabetes.
Similar content being viewed by others
Introduction
CHD is the major cause of mortality in subjects with type 2 diabetes [1, 2], and one of the key factors contributing to cardiovascular disease in type 2 diabetic patients is the impairment of lipid metabolism [3]. Several studies have shown that correction of dyslipidaemia significantly decreases the risk of CHD events [3, 4]. This may be achieved through use of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins), which can control both hypercholesterolaemia and hypertriglyceridaemia. Several clinical reports have shown that these agents reduce CHD and total mortality in diabetic subjects [5–7].
The retrospective analysis of data from the West of Scotland Coronary Prevention Study (WOSCOPS) showed that pravastatin therapy resulted in a 30% reduction in the hazard of developing diabetes [8]. On the other hand, it has been reported that atorvastatin increased the incidence of diabetes in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) [9], and that atorvastatin impaired glucose metabolism in some cases of type 2 diabetes [9, 10]. However, few studies have addressed the potential effects of statins upon glucose metabolism. Elucidation of the mechanisms underlying the pathological effects of statins on glucose metabolism is of importance, especially when deciding upon the most suitable of several statins for treatment of dyslipidaemia in type 2 diabetic patients. The adipocyte is one of the major targets for insulin action. During the course of insulin-stimulated glucose uptake in adipocytes, insulin activates the tyrosine kinase activity of the insulin receptor, which, in turn phosphorylates IRS-1, resulting in the recruitment of the insulin-sensitive solute carrier family 2 (facilitated glucose transporter), member 4 (SLC2A4, formerly known as GLUT4) to the plasma membrane and uptake of glucose [11]. The decreased expression of SLC2A4 contributes to insulin resistance and type 2 diabetes [12]. It was shown that TNFα, a factor that induces insulin resistance, decreased SLC2A4 expression in adipocytes [13], and that lovastatin administered at a super-pharmacological dose also decreased SLC2A4 expression [14]. Adipocytes influence glucose metabolism and type 2 diabetes, at least partly, by releasing adipocytokines that either impair or improve insulin action [15, 16]. Whether adipocytes exert beneficial or deleterious effects on glucose metabolism depends on the state of adipocyte differentiation [17].
In this study we aimed to clarify the effects of atorvastatin on adipocyte differentiation and SLC2A4 expression in 3T3-L1 cells and on glucose metabolism and SLC2A4 expression in NSY mice. This mouse reportedly exhibits moderate levels of obesity, insulin resistance, and impaired insulin response to glucose, thus displaying the characteristics of type 2 diabetes in humans. Furthermore, Japanese subjects with type 2 diabetes and hypercholesterolaemia were treated with atorvastatin, and its effects on glycaemic profiles were examined.
Subjects, materials and methods
Materials
All standard culture reagents were obtained from Gibco BRL (Grand Island, NY, USA). Pravastatin, atorvastatin and simvastatin were gifts from Sankyo (Tokyo, Japan). Pitavastatin was a gift from Kowa (Tokyo, Japan). Geranylgeranyl pyrophosphate (GGPP) and dl-mevalonic acid lactone (dl-mevalonate) were purchased from Sigma Chemical (St Louis, MO, USA).
Cell culture, induction of adipocyte differentiation and treatment with statins
The 3T3-L1 cells were maintained as previously described [18]. Differentiation of 3T3-L1 cells into adipocytes was induced by treatment of confluent monolayers for 2 days with 10 μg/ml insulin, 0.4 μg/ml dexamethasone and 0.5 mmol/l 3-isobutyl-1-methylxanthine (differentiation cocktail) in DMEM containing 10% foetal bovine serum (FBS), followed by subsequent culture to day 8 in most experiments. The day when this culture started was denoted as day 1. At day 3, the culture medium was changed to one containing 10% FBS and 10 μg/ml insulin. Insulin was removed on day 5, and the cells were fed every 2 days with DMEM containing 10% FBS.
To investigate the effects of HMG-CoA reductase inhibitors on adipocyte differentiation, the drug was added to the culture for the specific 2-day culture periods from day 1 to day 3 (days 1–3), days 3–5 or days 5–7. The cells on day 8 were used for most measurements. In the study to examine the effect of statins in the differentiated adipocytes, the cells were further cultured for 2 days (days 8–10) with or without atorvastatin at the indicated concentrations.
Cell culture, induction of myotube differentiation and treatment with statins
The C2C12 cells were cultured in DMEM with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in an atmosphere of 5% CO2, 95% air. Differentiation of C2C12 cells into myotubes was induced by treatment of confluent monolayers with 10 μg/ml insulin, 5 μg/ml transferrin, 10 nmol/l selenite and 1 mg/ml BSA in culture for 3 days.
Immunoblotting analysis
Treated cells were lysed in lysis buffer (100 mmol/l NaCl, 0.5% NP40, 1 mmol/l EDTA, 10 mmol/l Tris-HCl [pH 7.5], 0.5 U/ml aprotinin, 1 mmol/l PMSF). To extract nuclear proteins, cells were lysed in lysis buffer containing 1% SDS. For Akt phosphorylation, the cells were incubated with insulin (100 nmol/l) and lysed in lysis buffer containing 100 mmol/l sodium fluoride, 10 mmol/l sodium pyrophosphate, 2 mmol/l sodium orthovanadate. Cell lysates were subjected to SDS-PAGE through an 8 or 10% gel. SLC2A4 was detected with the SLC2A4 polyclonal antibody (a gift from Y. Oka, Tohoku University, Sendai, Japan). Hormone-sensitive lipase (HSL) was detected with the HSL polyclonal antibody [19]. IRβ, IRS-1, peroxisome proliferator-activated receptor γ (PPARγ) and caveolin-1 proteins were detected with anti-IR, anti-IRS-1, anti-PPARγ and anti-caveolin-1 primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Perilipin and CCAAT/enhancer binding protein α (C/EBPα) were detected with anti-perilipin and anti-C/EBPα primary antibodies (ABR—Affinity BioReagents, Golden, CO, USA). Fatty acid synthase (FAS) was detected with anti-FAS primary antibody (BD, San Jose, CA, USA). Phospho-Ser Akt was detected with the polyclonal antibody (CST, Beverly, MA, USA). Immunoreactive proteins were detected with HRP-conjugated secondary antibody and the ECL system (Amersham, Arlington Heights, IL, USA). For immunoblotting of Akt, membranes were reprobed and detected using anti-Akt polyclonal antibody.
Real-time RT-PCR analysis
Total RNA was isolated using TRIzol (Invitrogen, Tokyo, Japan), and first-strand cDNA synthesis was completed using the ReverTra Ace (Toyobo, Osaka, Japan). Using a QuantiTect SYBR Green PCR kit, real-time PCR was performed in an ABI-prism 7700 sequence detector (Applied Biosystems Japan, Tokyo, Japan). Using β-actin for internal control, data were analysed according to the 2−ΔΔCT method. Primers were as follows: β-actin, 5′-TTCCCCTCCATCGTGGGCCGC-3′ and 5′-GATGGCTACGTACATGGCTGG-3′; Slc2a4, 5′-CTTCTTTGAGATTGGCCCTGG-3′ and 5′-AGGTGAAGATGAA GAAGCCAAGC-3′; Cebpa, 5′-CCGGGAGAACTCTAACTC-3′ and 5′-GATGTAGGCGCTGATGT-3′.
Oil Red O staining and measurements of triglyceride content
At day 8, cells were fixed and stained with Oil Red O to morphologically assess adipocyte differentiation. The triglyceride content of lipid extracts was measured using an enzymatic assay kit (Triglyceride E-test; Wako, Osaka, Japan) and normalised to cell protein.
Measurement of 2-NBDG uptake
3T3-L1 cells were grown and differentiated with or without statins in 24-well culture plates. Before assaying, cells were preincubated with serum-free DMEM for 2 h. After washing with Krebs–Ringer phosphate buffer (KRB) containing 2.8 mmol/l glucose, cells were preincubated in 500 μl KRB for 30 min at 37°C. Cells were then stimulated with insulin (100 nmol/l) (Sigma) for 10 min in KRB at 37°C. Uptake was initiated by addition of a fluorescent derivative of 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose (2-NBDG) at 600 μmol/l in 2.8 mmol/l glucose containing KRB, as described previously [20]. After 15 min, the reaction was terminated by quickly washing with ice-cold KRB. Cells were lysed by freeze–thaw. Fluorescence of 2-NBDG was measured using fluorescence spectrophotometer F-2500 (Hitachi, Tokyo, Japan) at a wavelength of 540 nm (excitation wavelength 485 nm).
Tests of glucose metabolism in type 2 diabetes NSY mice
Male NSY mice weighing 25–28 g were purchased from Japan SLC (Hamamatsu, Japan) [21]. A single mouse was housed per cage, with a light–dark cycle of 12 h each (light from 07.00–19.00 h), and free access to food and water. From 5–20 weeks of age, NSY mice were given either a regular diet (CE-2; CLEA Japan, Tokyo, Japan) or one containing 0.05 g of atorvastatin per 100 g. All experimental procedures were carried out in accordance with the Japanese Physiological Society’s guidelines for animal care and approved by the institutional animal care and use committee. For glucose tolerance tests, 6-h-fasted NSY mice aged 20 weeks were injected i.p. with glucose (2.0 g/kg). For insulin tolerance tests, 6-h-fasted NSY mice aged 20 weeks were injected i.p. with rapid insulin (0.5 IU/kg). Glucose and insulin levels in blood samples obtained from the tail vein were determined by using Glucocard (Arkray, Kyoto, Japan) and ELISA (Shibayagi, Gumma, Japan), respectively.
Clinical research design
A total of 78 Japanese subjects with type 2 diabetes were recruited from the outpatients department of Jichi Medical University Hospital. Subjects were eligible for the study if they had been diagnosed with type 2 diabetes for at least 1 year. Other inclusion criteria were: (1) age 30–80 years; (2) stable glycaemic control; and (3) hypercholesterolaemia >5.17 mmol/l (200 mg/dl). The characteristics of subjects are shown in Table 1. Approximately 2 weeks after the initial screening, the patients were enrolled in the study if they still fulfilled the entrance criteria and gave their informed consent. They were all placed on atorvastatin (10 mg/day), and a lipid profile was obtained. Further assessments were made 12 weeks after initiation of therapy. Fasting blood samples were obtained for the measurement of serum lipid (total cholesterol, HDL cholesterol and triglycerides), glucose and HbA1c by standard laboratory techniques. Other therapeutic regimens—diet and exercise therapies, as well as glucose-lowering drugs—were kept unchanged throughout the observation period. The study was performed in accordance with the Declaration of Helsinki.
Statistical analyses
All data are presented as means±SEM (n=number of observations). The statistical analysis of experimental data was carried out with the Student’s t-test. For the clinical research study, paired results obtained at baseline and 12 weeks after initiation of atorvastatin medication were compared using the Wilcoxon matched-pairs signed-ranks test. Differences were considered statistically significant when the p value was less than 0.05.
Results
Effects of statins on differentiation of 3T3-L1 cells
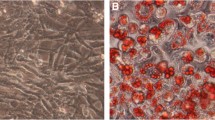
Addition of differentiation cocktail to the culture induced adipocyte differentiation in 3T3-L1 cells, as judged at day 8 of culture (Fig. 1a). We treated 3T3-L1 cells with statins for the indicated 2-day periods, covering the whole of the differentiation process. Treatment with 10 ng/ml atorvastatin from day 1 to day 3 (days 1–3) of culture had little influence on adipocyte differentiation (Fig. 1b). However, treatment with atorvastatin at days 3–5 of culture markedly inhibited adipocyte differentiation (Fig. 1c), while treatment at days 5–7 moderately suppressed it (Fig. 1d). In contrast, neither simvastatin nor pravastatin at 10 ng/ml during days 3–5 of culture had significant effects on adipocyte maturation (Fig. 1e,f), indicating that the inhibitory effect was specific to atorvastatin at this dose. The inhibitory effect of atorvastatin was concentration-dependent: the drug exerted a partial effect at 1 ng/ml, and near maximal effects at 10 and 100 ng/ml (Fig. 1g–i).
Effects of atorvastatin, simvastatin and pravastatin on adipocyte differentiation in 3T3-L1 cells. Cells were induced to differentiate by treatment with differentiation cocktail, as described in Subjects, materials and methods. At day 8 of culture, the cells were stained with Oil Red O and visualised under phase-contrast microscopy. Control cells (a) were not treated with statins. Cells were treated with 10 ng/ml atorvastatin at days 1–3 (b), days 3–5 (c) or days 5–7 (d) of culture. At days 3–5, other experiments were performed with 10 ng/ml simvastatin (e), 10 ng/ml pravastatin (f), or atorvastatin at concentrations varying from 1–100 ng/ml (g, h)
We next examined the effect of statins on the intracellular accumulation of lipids, a parameter associated with adipocyte maturation. Accumulation of triglycerides was inhibited by about 70% by treatment with atorvastatin (1–100 ng/ml) at days 3–5 of culture (Fig. 2a). This decrease in triglyceride content was reversed by the co-administration of l-mevalonate, the product of the reaction catalysed by HMG-CoA reductase. The inhibitory effect of atorvastatin was also reversed by GGPP, an intermediate in the HMG-CoA reductase pathway and the substrate for protein prenylation. Simvastatin, another lipophilic statin, had no effect on cellular triglyceride content at concentrations from 1 to 100 ng/ml, but at 1,000 ng/ml, significantly decreased it (Fig. 2b). Thus, simvastatin was approximately 1000 times less potent than atorvastatin. At concentrations ranging from 1–1,000 ng/ml, pravastatin, a hydrophilic statin, had no effect on intracellular triglyceride levels.
Effects of statins on triglyceride content, levels of proteins involved in insulin signalling, and glucose uptake in 3T3-L1 cells. a Treatment with atorvastatin at days 3–5 of culture decreased intracellular triglyceride content in 3T3-L1 cells as judged at day 8. This decrease in triglyceride content was reversed by co-administration of 100 μmol/l l-mevalonate (MVA) or 10 μmol/l GGPP. *p<0.05 vs control. b Effects of treatment with simvastatin (black bars) or pravastatin (grey bars) at days 3–5 of culture on intracellular triglyceride content. *p<0.05 vs control. c Effects of atorvastatin treatment at days 3–5 of culture on levels of the adipocyte differentiation-related proteins IRβ, IRS-1, SLC2A4 and HSL. Atorvastatin suppressed IRS-1 and SLC2A4 and enhanced HSL. Inhibitory effects of atorvastatin were prevented by the presence of 100 μmol/l l-mevalonate or 10 μmol/l GGPP at days 3–5. d Effects of treatment with atorvastatin (ATR), simvastatin (SIM) or pravastatin (PRA) at days 3–5 of culture on insulin-induced glucose uptake. Atorvastatin reduced glucose uptake. *p<0.05 vs control
Effects of atorvastatin on levels of insulin signalling-associated and/or adipocyte-associated proteins and glucose uptake in 3T3-L1 cells during the adipocyte differentiation period
Treatment with atorvastatin at days 3–5 also influenced the level of several proteins (Fig. 2c), downregulating IRS-1 and SLC2A4 markedly and IRβ slightly, while upregulating HSL. The effects of atorvastatin on IRβ, IRS-1 and SLC2A4 levels were blocked when mevalonate or GGPP was co-administered in the media (Fig. 2c), suggesting that the inhibitory effects were the result of blockade of HMG-CoA reductase and isoprenoid biosynthesis. The glucose uptake induced by 100 nmol/l insulin in 3T3-L1 cells was significantly reduced by treatment with 1–100 ng/ml atorvastatin during days 3–5 of the culture period (Fig. 2d). In contrast, simvastatin and pravastatin had no effect on glucose uptake. These results suggest that atorvastatin inhibits adipocyte maturation. In addition, treatment with atorvastatin markedly reduced levels of adipocyte-associated proteins, FAS, perilipin and caveolin-1; these effects were counteracted by co-administration of GGPP (Fig. 3a). Furthermore, insulin-induced Akt activation was suppressed in cells treated with atorvastatin (Fig. 3b). Suppression of insulin-induced Akt activation and glucose uptake was blocked by co-administration of GGPP (Fig. 3b,c). Several transcription factors are known to co-operate in the adipocyte differentiation process; PPARγ and C/EBPα are implicated in the regulation of SLC2A4 expression. Treatment with atorvastatin also markedly reduced PPARγ and C/EBPα levels as judged at day 5 of culture, and these changes were partially counteracted by GGPP (Fig. 3d). The expression of Slc2a4 and Cebpa mRNA was markedly decreased in cells treated with atorvastatin as judged at day 5 of culture; these effects were blunted when GGPP was co-administered (Fig. 4a). In contrast, when added to C2C12 cells in the culture to induce differentiation into mytubes, atorvastatin failed to affect levels of Slc2a4 mRNA or the SLC2A4 protein (Fig. 4b,c).
Effects of atorvastatin on levels of adipocyte differentiation-related proteins and insulin-signalling in 3T3-L1 cells. a Treatment with atorvastatin (ATR; 100 ng/ml) at days 1–8 of culture reduced the levels of the adipocyte differentiation-related proteins FAS, perilipin and caveolin-1 as judged at day 8. These effects were partially (for FAS and perilipin) or fully (for caveolin-1) counteracted by co-administration of 10 μmol/l GGPP. b Atorvastatin treatment at days 1–8 of culture reduced Akt phosphorylation (P-Akt) induced by insulin (100 nmol/l), and this effect was prevented by co-administration of 10 μmol/l GGPP. c Atorvastatin treatment at days 1–8 of culture reduced insulin (100 nmol/l)-induced glucose uptake, and this effect was partially counteracted by co-administration of 10 μmol/l GGPP. *p<0.05 vs other groups. d Atorvastatin treatment at days 1–5 of culture reduced levels of the adipocyte differentiation-related transcription factors PPARγ and C/EBPα, and these effects were partially prevented by co-administration of 10 μmol/l GGPP
Effects of atorvastatin treatment on Slc2a4 and Cebpa mRNA expression in adipocytes and muscle cells during differentiation. a Treatment with 100 ng/ml atorvastatin at days 1–5 of culture significantly decreased levels of Slc2a4 and Cebpa mRNA in 3T3-L1 cells as judged at day 5. *p<0.05 vs control. Treatment with 100 ng/ml atorvastatin at days 1–4 of culture had no effect on Slc2a4 mRNA (b) and SLC2A4 protein levels (c) in C2C12 cells as judged at day 4. Values are expressed as fold changes relative to the control without atorvastatin in a and b
Effects of atorvastatin on levels of insulin signalling-associated and/or adipocyte-associated proteins and glucose uptake in differentiated 3T3-L1 cells
The observed changes in protein levels could be the result of inhibition of adipocyte differentiation by atorvastatin. To examine an alternative or additional mechanism, we investigated the effect of atorvastatin on protein levels in the already differentiated 3T3-L1 cells at days 8–10 of culture. The SLC2A4 level was reduced by treatment with 1–100 ng/ml atorvastatin, and this effect was prevented when mevalonate or GGPP was co-administered (Fig. 5a). In contrast, only the highest concentration of atorvastatin (100 ng/ml) increased levels of IRβ, IRS-1 and HSL. Simvastatin also reduced SLC2A4 levels, but only at a concentration of 100 ng/ml, while 1–100 ng/ml pravastatin had no effect (Fig. 5b). Treatment with 1–100 ng/ml atorvastatin significantly reduced the glucose uptake induced by 100 nmol/l insulin (Fig. 5c), though the magnitude of the reduction was smaller than that observed in differentiating cells (Fig. 2d). Glucose uptake tended to be reduced by 100 ng/ml simvastatin, but was unaffected by pravastatin and pitavastatin at the concentrations tested (Fig. 5c,d). GGPP partially counteracted the inhibitory effect of atorvastatin (Fig. 5e). Treatment with atorvastatin had no effect on caveolin-1 levels or the insulin-induced phosphorylation of Akt (Fig. 6a,b). Furthermore, atorvastatin also markedly decreased C/EBPα levels, but not PPARγ levels, (Fig. 6c), and this decrease was partially restored by co-treatment with GGPP (Fig. 6c). The expression of Slc2a4 and Cebpa mRNA was markedly decreased in the cells treated with atorvastatin, and these effects were blunted when GGPP was co-administered (Fig. 6d). Thus, both differentiating and fully differentiated 3T3-L1 cells showed decreased SLC2A4 and C/EBPα levels in response to atorvastatin treatment.
Effects of atorvastatin on the levels of insulin signalling-related proteins and glucose uptake in differentiated 3T3-L1 cells. a Effects of atorvastatin treatment on levels of IRβ, IRS-1, SLC2A4 and HSL at days 8–10 of culture, at which time adipocyte differentiation had been completed. Atorvastatin at 1–100 ng/ml selectively reduced SLC2A4 levels as judged at day 10, and this effect was prevented when 100 μmol/l l-mevalonate (MVA) or 10 μmol/l GGPP was added. b Effects on SLC2A4 levels of treatment with 1 ng/ml atorvastatin (ATR), 100 ng/ml simvastatin (SIM) or 100 ng/ml pravastatin (PRA) at days 8–10 of culture. Atorvastatin and simvastatin decreased SLC2A4 expression relative to the control (Cont). c, d Effects of treatment with statins on insulin-induced glucose uptake at days 8–10 of culture. Insulin (100 nmol/l)-induced glucose uptake was significantly reduced by atorvastatin at 1 and 100 ng/ml and tended to be reduced by simvastatin at 100 ng/ml, but was unaffected by treatment with 100 ng/ml pravastatin or 100 ng/ml pitavastatin. *p<0.05 vs control. e The inhibitory effect of atorvastatin (100 ng/ml) on insulin-induced glucose uptake was partially impeded by co-administration of 10 μmol/l GGPP at days 8–10. *p<0.05 vs other groups
Effects of atorvastatin on levels of adipocyte differentiation-related proteins, insulin signalling and Slc2a4 and Cebpa expression in differentiated 3T3-L1 cells. Treatment with 100 ng/ml atorvastatin at days 8–10 of culture influenced neither the caveolin-1 level (a) nor insulin-induced Akt phosphorylation (P-Akt) (b) as judged at day 10. c Atorvastatin treatment at days 8–10 of culture reduced the level of C/EBPα, but not PPARγ, at day 10. d Treatment with 100 ng/ml atorvastatin at days 8–10 of culture significantly decreased Slc2a4 and Cebpa mRNA expression at day 10. The inhibitory effects of atorvastatin were counteracted by co-administration of 10 μmol/l GGPP at days 8–10. *p<0.05 vs other groups
Effects of atorvastatin on glucose metabolism and SLC2A4 expression in NSY mice
Based on the above observation that atorvastatin attenuated adipocyte differentiation and SLC2A4 expression in vitro, we next examined the possible impact of these effects on in vivo glucose metabolism using NSY mice. This mouse reportedly exhibits moderate levels of obesity, insulin resistance and impaired insulin response to glucose [21], and is therefore considered a model for human type 2 diabetes. We placed NSY mice on regular diet (control group) or one containing atorvastatin (ATR group) from 5–20 weeks of age. Body weight and fasted plasma glucose were not significantly different between the two groups over this period (data not shown). However, inter-group differences were observed when the glucose tolerance test was administered: in the ATR group, blood glucose levels at 30 and 60 min after glucose injection were higher and the recovery toward baseline was delayed compared with those for the control group (Fig. 7a). The AUC was significantly larger for the ATR group than the control group (Fig. 7b). The plasma insulin concentrations, both in the fasting state and following glucose load were not significantly different between control and ATR groups (Fig. 7c). During the insulin tolerance test, blood glucose levels at 60 and 90 min after insulin injection were significantly higher in the ATR group, indicating reduced insulin sensitivity (Fig. 7d). These results indicate that treatment with atorvastatin affects glucose metabolism mainly via inducing insulin resistance. Furthermore, treatment with atorvastatin reduced levels of SLC2A4, but not HSL, in the retroperitoneal white adipose tissue (WAT) of NSY mice (Fig. 8).
Effects of treatment with atorvastatin on glucose and insulin levels in NSY mice. a Results of a glucose tolerance test (GTT) in NSY mice fed for 15 weeks with a regular diet (Cont group; open circles) or one containing atorvastatin (ATR group; closed cycles). Plasma glucose levels at the indicated time points are expressed as means±SEM, n=7 for each group. b Increases in plasma glucose levels during the GTT were shown by integrated AUCs over the first 2 h after glucose administration, and expressed as means±SEM, n=7 for each group. *p<0.05 vs Cont group. c Plasma insulin concentrations prior to and 15 min after glucose administration. Bars represent means±SEM, n=7 for each group. No significant differences were observed between the two groups. d Results of an insulin tolerance test (ITT) in Cont. (open circles) and ATR groups (closed circles). Plasma glucose concentrations at the indicated time points were expressed as fold changes relative to initial values and shown as means±SEM, n=5 for each group. *p<0.05 vs Cont group
Clinical study
As shown in Table 2, atorvastatin significantly decreased serum total cholesterol and triglycerides without altering serum HDL cholesterol. Atorvastatin treatment was followed by a significant increase in HbA1c, while fasting plasma glucose remained unchanged. The increase in HbA1c was more prominent among the subgroup of non-obese patients.
Discussion
This study demonstrates that treatment with atorvastatin at clinical doses inhibits adipocyte differentiation, decreases SLC2A4 expression in both differentiating and mature adipocytes, and impairs insulin sensitivity and post-challenge glucose tolerance in an animal model of type 2 diabetes. In type 2 diabetic patients, particularly in those without obesity, atorvastatin treatment was followed by an increase in HbA1c.
In this study, differentiation of 3T3-L1 cells into adipocytes was induced, and the stage of differentiation/maturation was judged by measurement of adipocyte markers and markers of insulin signalling; these included oil droplet formation, triglyceride accumulation, levels of SLC2A4, FAS, perilipin and caveolin-1, and insulin-induced glucose uptake and Akt phosphorylation. Atorvastatin administered at days 3–5 of culture markedly reduced all these parameters.
PPARγ and C/EBPα co-operate to induce the production of adipocyte-specific proteins, such as perilipin and SLC2A4, during the late stages of adipocyte differentiation, resulting in the accumulation of intracellular lipids and promotion of insulin-stimulated SLC2A4 translocation and glucose uptake [22]. In this study, atorvastatin suppression of PPARγ and C/EBPα levels appeared at day 5 of culture, while suppression of SLC2A4 and caveolin-1 levels was observed at day 8 of culture. We therefore suggest that the atorvastatin-induced decrease in PPARγ and C/EBPα levels during the early stages of differentiation is at least partly responsible for the subsequent reduction in SLC2A4 and caveolin-1 levels at late stages of differentiation. In adipocytes, the insulin receptor is localised in caveolae, and insulin stimulation translocates SLC2A4 to caveolae, a process implicated in glucose uptake [23]. Therefore, suppression of SLC2A4 and caveolin-1 expression by atorvastatin may be linked to the reduction in the insulin-stimulated glucose uptake.
These inhibitory effects of atorvastatin were counteracted by the co-administration of mevalonate or GGPP, indicating that atorvastatin inhibits adipocyte differentiation by inhibiting HMG-CoA reductase. These effects of atorvastatin were maximal when administered at days 3–5 of culture, in accordance with a previous report that the expression of cholesterol metabolic enzymes including HMG-CoA reductase is remarkably increased at day 3 of differentiation of 3T3-L1 cells [24]. When administered after differentiation (on days 8–10), atorvastatin suppressed C/EBPα and SLC2A4 levels and insulin-induced glucose uptake. The inhibition of SLC2A4 expression in adipocytes by atorvastatin, both during and after differentiation, was reversed by GGPP. Inhibition of isoprenoid biosynthesis is associated with the reduced expression of SLC2A4 [25]. C/EBPα directly binds to the Slc2a4 promoter to induce expression of the gene [26], maintaining insulin-stimulated SLC2A4 translocation [27]. Thus, atorvastatin may reduce SLC2A4 expression primarily via inhibition of the synthesis of isoprenoid cholesterol precursors and downregulating C/EBPα production.
The effective concentrations of atorvastatin observed in this study are consistent with those reported in the plasma of patients treated with this drug [28]. Simvastatin also suppressed adipocyte differentiation, but only at 1,000 ng/ml, which is 2–4 logarithmic orders higher than its plasma concentration in patients receiving this drug [29, 30]. Pravastatin had little effect on adipocyte differentiation. Therefore, the suppression of adipocyte differentiation observed in this study may not occur in patients treated with simvastatin or pravastatin. The lack of effect of pravastatin may be due to its hydrophilicity. The reason why atorvastatin is more potent than simvastatin in inhibiting the differentiation of adipocytes and SLC2A4 expression in these cells remains to be clarified. However, the simplest explanation is that adipocytes are more permeable to atorvastatin, giving this agent easier access to HMG-CoA reductase [28]. We previously reported that simvastatin inhibited insulin secretion by interacting with rat islet beta cells in vitro [31]. In the present study, atorvastatin had little influence on insulin secretion in vivo in NSY mice. These results suggest that beta cells have a higher affinity for simvastatin than for atorvastatin, although it should be noted that there were differences in experimental conditions between the two studies. Thus, the effects of statins could be produced through relatively selective interaction between the specific statin and the specific target tissue/cell.
In type 2 diabetic NSY mice, oral administration of atorvastatin impaired glucose tolerance, primarily as a result of induction of insulin resistance. Furthermore, atorvastatin reduced SLC2A4 levels in the adipocytes of NSY mice. Our finding is in accordance with the report that the adipose tissue-specific decrease in SLC2A4 expression causes insulin resistance not only in adipocytes but also in muscle and liver [32]. Thus, the downregulation of SLC2A4 in adipocytes can lead to insulin resistance of the whole body and accelerate type 2 diabetes [32], which may occur in the atorvastatin-administered NSY mice.
In this study, treatment with atorvastatin was followed by an increase in HbA1c, but not fasting plasma glucose levels, in Japanese patients with type 2 diabetes, and this effect was more prominent in non-obese patients. These results suggest that atorvastatin treatment may impair postprandial glucose levels; however, these results are limited by the fact that there was no control group. This finding is consistent with a recent study on patients with hypercholesterolaemia, in which the HbA1c level was significantly increased in the atorvastatin-treated, but not the pravastatin-treated, group [33]. Japanese non-obese type 2 diabetic patients are often characterised by a more prominent impairment in insulin secretion than insulin action [34]. Therefore, we suggest a scenario in which atorvastatin inhibits adipocyte functions, thereby inducing and/or enhancing insulin resistance, and impairs post-prandial glucose tolerance; these effects could influence glycaemic control in diabetes, especially in non-obese patients, who are characteristically associated with impaired insulin secretion. In the obese subgroup of type 2 diabetic patients, the influence of atorvastatin on glycaemic control was less pronounced. It is widely accepted that elevation of the triglyceride-rich lipoproteins associated with obesity accelerates glucose intolerance, a process known as lipotoxicity [35]. Several clinical studies have reported that the statins used in the current study all effectively lower plasma levels of both total and LDL cholesterol, and raise plasma HDL cholesterol levels, while atorvastatin is distinctly stronger in lowering the plasma triglyceride concentration [36]. In obese type 2 diabetic patients with hypertriglyceridaemia, a favourably greater ability of atorvastatin to lower triglycerides could result in an effective correction of lipotoxicity, and could therefore help maintain glycaemic control. The anti-inflammatory action of statins could also act against diabetes [37]. These beneficial effects of atorvastatin could counteract possible negative side effects associated with this drug.
Taken together, atorvastatin appears to influence multiple processes involved in glucose metabolism; these changes have both beneficial and disadvantageous effects. Their balance and net outcome may be determined by the metabolic conditions of individuals and, in turn, influence whether, overall, this drug corrects or worsens insulin resistance and, ultimately, type 2 diabetes. Since a large number of diabetic patients with diverse metabolic conditions are treated with statins over a long period, and adipocytes play a central role in regulating glucose and lipid metabolism, atorvastatin’s inhibitory effects on adipocytes and their possible impact on the glycaemic control should be kept in mind.
Abbreviations
- C/EBP:
-
CCAAT/enhancer binding protein
- FAS:
-
fatty acid synthase
- GGPP:
-
geranylgeranyl pyrophosphate
- HMG-CoA:
-
3-hydroxy-3-methylglutaryl-coenzyme A
- HSL:
-
hormone-sensitive lipase
- IRβ:
-
insulin receptor β-subunit
- 2-NBDG:
-
2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose
- PPAR:
-
peroxisome proliferator-activated receptor
- SLC2A4:
-
solute carrier family 2 (facilitated glucose transporter), member 4
References
Kannel WB, McGee DL (1979) Diabetes and cardiovascular disease: the Framingham study. JAMA 241:2035–2038
Turner RC, Millns H, Neil HA et al (1998) Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 316:823–828
Shepherd J, Cobbe SM, Ford I et al (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolaemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 333:1301–1307
Heart Protection Study Collaborative Group (2002) MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360:7–22
Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G (1997) Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease: a subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 20:614–620
Goldberg RB, Mellies MJ, Sacks FM, et al (1998) Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the Cholesterol and Recurrent Events (CARE) trial: the Care Investigators. Circulation 98:2513–2519
Yeo WW, Yeo KR (2001) Predicting CHD risk in patients with diabetes mellitus. Diabet Med 18:341–344
Freeman DJ, Norrie J, Sattar N et al (2001) Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 103:357–362
Sever PS, Dahlof B, Poulter NR et al (2003) Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361:1149–1158
Diabetes Atorvastin Lipid Intervention (DALI) Study Group (2001) The effect of aggressive versus standard lipid lowering by atorvastatin on diabetic dyslipidemia: the DALI study: a double-blind, randomized, placebo-controlled trial in patients with type 2 diabetes and diabetic dyslipidemia. Diabetes Care 24:1335–1341
Khan AH, Pessin JE (2002) Insulin regulation of glucose uptake: a complex interplay of intracellular signalling pathways. Diabetologia 45:1475–1483
Garvey WT, Maianu L, Huecksteadt TP, Birnbaum MJ, Molina MJ, Ciaraldi TP (1991) Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest 87:1072–1081
Stephens JM, Lee J, Pilch PF (1997) Tumor necrosis factor-α-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem 272:971–976
Chamberlain LH (2001) Inhibition of isoprenoid biosynthesis causes insulin resistance in 3T3-L1 adipocytes. FEBS Letters 507:357–361
Matsuzawa Y, Shimomura I, Kihara S, Funahashi T (2003) Importance of adipocytokines in obesity-related diseases. Horm Res 60(Suppl 3):56–59
Matsuzawa Y, Funahashi T, Kihara S, Shimomura I (2004) Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 24:29–33
Kadowaki T, Hara K, Yamauchi T, Terauchi Y, Tobe K, Nagai R (2003) Molecular mechanism of insulin resistance and obesity. Exp Biol Med 228:1111–1117
Nakata M, Yada T, Shioda S, Oka Y, Maruyama I (1999) Insulinotropin PACAP potentiates insulin-stimulated glucose uptake in 3T3-L1 cells. Peptides 20:943–948
Okazaki H, Osuga J, Tamura Y et al (2002) Lipolysis in the absence of hormone-sensitive lipase: evidence for a common mechanism regulating distinct lipases. Diabetes 51:3368–3375
Yamada K, Nakata M, Horimoto N, Saito M, Matsuoka H, Inagaki N (2000) Measurement of glucose uptake and intracellular calcium concentration in single, living pancreatic β-cells. J Biol Chem 275:22278–22283
Ueda H, Ikegami H, Yamato E (1995) The NSY mouse: a new animal model of spontaneous NIDDM with moderate obesity. Diabetologia 38:503–508
Wu Z, Rosen ED, Brun R et al (1999) Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell 3:151–158
Cohen AW, Combs TP, Scherer PE, Lisanti MP. (2003) Role of caveolin and caveolae in insulin signaling and diabetes. Am J Physiol Endocrinol Metab 285:E1151–E1160
Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS (1997) Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest 99:838–845
Klemm DJ, Leitner JW, Watson P et al (2001) Insulin-induced adipocyte differentiation. Activation of CREB rescues adipogenesis from the arrest caused by inhibition of prenylation. J Biol Chem 276:28430–28435
Fujimoto M, Masuzaki H, Yamamoto Y et al (2005) CCAAT/enhancer binding protein α maintains the ability of insulin-stimulated GLUT4 translocation in 3T3-C2 fibroblastic cells. Biochim Biophys Acta 1745:38–47
Kaestner KH, Christy RJ, Lane MD (1990) Mouse insulin-responsive glucose transporter gene: characterization of the gene and transactivation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci USA 87:251–255
Schmitz G, Drobnik W (2003) Pharmacogenomics and pharmacogenetics of cholesterol-lowering therapy. Clin Chem Lab Med 41:581–589
Lilja JJ, Kivisto KT, Neuvonen PJ (1999) Grapefruit juice increases serum concentrations of atorvastatin and has no effect on pravastatin. Clin Pharmacol Ther 66:118–127
Lilja JJ, Kivisto KT, Neuvonen PJ (1998) Grapefruit juice–simvastatin interaction: effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin Pharmacol Ther 64:477–483
Yada T, Nakata M, Shiraishi T, Kakei M (1999) Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet β-cells. Br J Pharmacol 126:1205–1213
Abel ED, Peroni O, Kim JK et al (2001) Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409:729–733
Ishikawa M, Namiki A, Kubota T et al (2006) Effect of pravastatin and atorvastatin on glucose metabolism in nondiabetic patients with hypercholesterolemia. Intern Med 45:51–55
Yoshinaga H, Kosaka K (1999) Heterogeneous relationship of early insulin response and fasting insulin level with development of non-insulin-dependent diabetes mellitus in non-diabetic Japanese subjects with or without obesity. Diabetes Res Clin Pract 44:129–136
DeFronzo RA, Ferrannini E (1991) Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14:173–194
Bakker-Arkema RG, Davidson MH, Goldstein RJ et al (1996) Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA 275:128–133
Economides PA, Caselli A, Tiani E, Khaodhiar L, Horton ES, Veves A (2004) The effects of atorvastatin on endothelial function in diabetic patients and subjects at risk for type 2 diabetes. J Clin Endocrinol Metab 89:740–747
Acknowledgements
We thank T. Miyoshi and S. Usui for expert technical assistance. This study was supported by grants from a Grant-in-Aid for Scientific Research (to M. Natkata, T. Yada, S. Ishibashi) and a Grant-in-Aid for Scientific Research on Priority Areas (15081101) (to T. Yada) from the Japan Society for the Promotion of Science, a grant from the 21st Century Center of Excellence program (to T. Yada, S. Ishibashi), and an Insulin Research Award from Novo Nordisk Pharma (to T. Yada).
Duality of interest
This work was supported in part by a research grant from Sankyo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakata, M., Nagasaka, S., Kusaka, I. et al. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia 49, 1881–1892 (2006). https://doi.org/10.1007/s00125-006-0269-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0269-5