Abstract
Aims/hypothesis
Adiponectin is an adipokine that exhibits insulin-sensitising, fat-burning and anti-inflammatory properties as well as modulatory effects on oxidative stress. We examined whether adiponectin could be induced in a non-adipose tissue, skeletal muscle, in response to metabolic or oxidative aggression both in vivo (in a murine model of type 2 diabetes) and in vitro.
Methods
Obese and diabetic ob/ob mice were used and compared with lean littermates. Some obese mice were treated with the antioxidant probucol for 3 weeks. At the end of the experiment, blood was sampled and tibialis anterior muscles were collected for mRNA measurement and immunohistochemistry. Additional in vitro experiments were performed on C2C12 myotubes cultured for up to 48 h.
Results
In spite of hypoadiponectinaemia, Adipoq mRNA levels were markedly increased in the skeletal muscle of ob/ob mice and correlated with systemic oxidative stress. Adipoq upregulation was shown in laser-microdissected myocytes of obese mice. Concomitantly, immunoreactivity for adiponectin was enhanced in obese muscle fibres together with lipid infiltration and local markers of oxidative stress. In cultured C2C12 myotubes, a triglyceride mix and reactive oxygen species producers (H2O2 or a lipoperoxidation end-product) upregulated Adipoq expression and adiponectin production. This effect was reversed by an antioxidant. Finally, treatment of obese mice with probucol also attenuated upregulation in muscle.
Conclusions/interpretation
The paradoxical upregulation of adiponectin in muscle of obese and diabetic mice may result from lipotoxicity and related oxidative stress. This unexpected finding could be viewed as a local protection to counteract ectopic fat deposition and oxidative damage.
Similar content being viewed by others
Introduction
Adiponectin (whose gene is referred to as Adipoq) is an adipokine that plays a fundamental role in energy homeostasis and inflammation [1]. It is composed of an N-terminal collagenous domain and a C-terminal globular domain. The latter fragment, globular adiponectin, generated by proteolysis, may exert some biological effects by itself and often proves to be far more potent than full-length adiponectin on muscle [2, 3]. This may be explained by the relative abundance of the two types of adiponectin receptor in this tissue. Adiponectin receptor 1 (Adipor1), which is a high-affinity receptor for globular adiponectin, is most abundantly expressed in skeletal muscle, while adiponectin receptor 2 (Adipor2), which serves as a moderate-affinity receptor for both forms of adiponectin, is predominant in liver [4].
Adiponectin exhibits insulin-sensitising properties on liver and muscle in vivo and in vitro [5, 6]. It increases glucose uptake by C2C12 myocytes or isolated mouse muscle [3, 7]. Adiponectin also alters lipid metabolism, by stimulating fatty acid oxidation in several tissues including muscle, thereby accelerating the clearance of plasma NEFA [2, 3, 7, 8]. Most of these effects are mediated by stimulation of AMP kinase and peroxisome proliferator-activated receptor (PPAR)-α ligand activities [4, 9]. Eventually, adiponectin exerts anti-atherogenic and anti-inflammatory properties as well as a protective role against oxidative stress [10–13]. It inhibits the inflammatory response to TNF-α and superoxide generation in endothelial cells [11, 14].
Further support for the metabolic and anti-atherogenic effects of adiponectin comes from clinical studies. Thus, plasma adiponectin is decreased in human subjects with obesity, type 2 diabetes or cardiovascular disease [11, 15–17]. Circulating adiponectin is also negatively correlated with systemic oxidative stress, independently of C-reactive protein, adiposity and glucose-tolerance status [13, 18]. This is of importance since oxidative stress may be involved in type 2 diabetes by promoting insulin resistance and decreasing insulin secretion, as well as in cardiovascular disease and hypertension by directly affecting vascular cell walls [18]. Circulating adiponectin is now proposed as a biomarker of the metabolic syndrome [19] and oxidative stress as a critical instigator of this syndrome [18].
To date, adiponectin regulation has been nearly exclusively studied in the adipocyte, the unique site of its production under normal conditions [20]. However, we have recently shown that adiponectin can be induced in mouse skeletal muscle and cultured myotubes in response to lipopolysaccharide (LPS) or a pro-inflammatory cytokine combination [21].
The aim of the present work was to examine whether adiponectin could also be induced in muscle in response to metabolic and/or oxidative aggression both in vivo, in a murine model of type 2 diabetes, and in vitro, in cultured myotubes. We used leptin-deficient ob/ob mice as a model of obesity and type 2 diabetes.
Materials and methods
Animals
The University Animal Care Committee approved all procedures. Female C57BL/6J obese (ob/ob) and lean littermate (+/?) mice were purchased from Charles River Laboratories (Brussels, Belgium) and studied from the age of 13 weeks. They received a free supply of common laboratory chow in powdered form (R04-10; UAR, Villemoisson-sur-Orge, France).
The animals were divided into three experimental groups: lean mice (n=10), untreated obese mice (n=5) and obese mice treated with probucol (n=5). Probucol (MP Biomedicals, Eschwege, Germany) was homogeneously added to food (0.5% w/w) as described [22] and administered for 3 weeks. At the beginning of the study, the two groups of ob/ob mice were matched for body weight and post-feeding blood glucose (Fig. 1a,c).
Body weight (a), food consumption (b), blood glucose (c) and plasma TBARS levels (d). Three groups of mice were studied: lean (L, filled squares); untreated ob/ob (O, open circles); and ob/ob treated with probucol (P, open triangles) for 3 weeks. All measurements or samplings were made between 08.00 and 09.00 h at the indicated period. Plasma was sampled at the end of the study (3 weeks), and TBARS, a marker of oxidative stress, was measured by a colorimetric method. Values are means±SEM for ten, five and five mice in the respective groups. **p<0.01 vs L mice
Body weight and food intake were measured daily. On several occasions, tail vein blood was collected from fed mice (between 08.00 and 09.00 h). At the end of the experiment, the mice were killed by decapitation (between 09.00 and 10.30 h), and larger blood samples were saved. Tibialis anterior muscle and inguinal fat were dissected, weighed, frozen in liquid nitrogen and stored at −80°C.
Light microscopy and immunohistochemistry
Muscle samples were fixed in 10% formaldehyde for 24 h and embedded in paraffin. Five-micron-thick sections were stained with haematoxylin–eosin–safran (HES). For immunohistochemistry, sections were processed as previously described [23], using rabbit polyclonal antibodies directed against adiponectin (Chemicon, Biognast, Hevle, Belgium), PPARγ or caspase-6 (both from Tebu-Bio, Baeckout, Belgium), peroxiredoxin 3 (PRDX3) or peroxiredoxin 5 (PRDX5) (gifts from B. Knoops, University of Louvain, Brussels, Belgium [24]) or a mouse monoclonal antibody directed against myosin (DSMZ, Braunschweig, Germany). Antibody concentrations and incubation times were 1 μg/ml for 48 h (adiponectin), 4 μg/ml for 48 h (PPARγ), 10 μg/ml overnight (caspase-6 and myosin), 5 μg/ml for 1 h (PRDX3) and 2 μg/ml for 1 h (PRDX5). The sections used for adiponectin, PPARγ and caspase-6 were pretreated in a microwave oven in Tris–citrate buffer (pH 6.5) for one cycle of 3 min at 750 W and three cycles of 3.5 min at 350 W. Binding of antibodies was detected by applying for 30 min at room temperature a second antibody, which was a goat anti-rabbit or anti-mouse immunoglobulin conjugated to peroxidase-labelled polymer (En Vision +; Dako, Copenhagen, Denmark). Peroxidase activity was revealed with 3-amino-9-ethylcarbazole substrate (Dako), which produces a red stain. Immunohistochemical controls were performed by omission of the first antibody or of the first and second antibodies, by using pre-immune serum, or by incubation with an irrelevant antibody (anti-thyroglobulin).
Morphometry
The mean relative proportion of myocyte and adipocyte areas was estimated by a point-counting technique [25] at ×50 magnification on paraffin sections of tibialis anterior muscle.
Oil Red O staining
Muscle samples were embedded in Tissue-Tek and rapidly frozen in isopentane cooled in liquid nitrogen to generate 5-μm-thick cryostat sections. Unfixed frozen sections were then treated as described [26].
Laser microdissection and pressure catapulting
Microdissection was used to selectively capture myocytes from frozen muscle. Seven-micron-thick sections were performed on muscle samples of two lean and two obese mice and laid on PEN membrane-coated slides. Immediately after dehydration, areas of intact myocytes (700,000 μm2 for each mouse) were microdissected with a PALM Microlaser system (Bernried, Germany) equipped with a pulsed laser (nitrogen, 337 nm) and the PALM Robot Software Version 2.2. This system was coupled to an Axiovert 200 microscope and a Plan Neofluar 20X (Zeiss, Oberkochen, Belgium). Then, myocytes were catapulted into an RNase-free microtube cap and frozen until RNA extraction.
Cell culture
C2C12 myoblasts were cultured as previously described [21]. At the time-zero point (96 h differentiation), different agents were added to the basal medium (high-glucose DMEM+2% heat-inactivated horse serum) alone or in combination for up to 48 h. We prepared AGE as described [27], and purchased 4-hydroxy-2-nonenal (HNE) (Calbiochem, VWR International, Zaventem, Belgium), recombinant murine leptin (Peprotech, London, UK), N-acetylcysteine (NAC) (Merck, VWR International), Structolipid 20% (structured triglycerides containing randomly esterified medium and long chain fatty acids; Fresenius Kabi, Bad Homburg, Germany), and other high-grade agents. The concentrations of the agents used were similar to those reported by others and devoid of overt cytotoxicity [27, 28]. HNE and cholesterol were dissolved in ethanol. In this case, the control conditions included the same amount of ethanol. At the end of the culture, aliquots of medium were saved and stored at −20°C and the cells rinsed twice in PBS before RNA isolation.
RNA extraction and real-time quantitative PCR (RTQ-PCR)
RNA was isolated from mouse tissues and cultured cells with TriPure (Roche Diagnostics, Vilvoorde, Belgium) or from laser-microdissected samples with an RNAqueous-Micro isolation kit (Ambion, Huntingdon, UK). Two micrograms of total RNA (or the whole amount extracted from microdissected samples) were reverse transcribed as described previously [21]. RTQ-PCR primers were designed (Primer Express Software; Applied Biosystems) for mouse adipocyte P2 (Fabp4), Adipoq, Adipor1, Adipor2, cyclophilin (Ppia), Pparγ, Prdx3, Prdx5 and Tnf (Table 1). One hundred and twenty nanograms of total RNA equivalents (or the whole amount obtained for microdissected samples) were amplified using an iCycler iQ Real Time PCR System (Bio-Rad Laboratories, Belgium) [21].
Quantification of adiponectin and circulating parameters
Adiponectin concentrations were measured with an RIA (kit from Linco Research, St Charles, MO, USA) in mouse plasma and homogenates of C2C12 myotubes, as reported previously [21]. Lipid peroxidation was assessed by measuring thiobarbituric acid reactive substance (TBARS) formation [29] and plasma 8-isoprostanes by an enzyme immunoassay kit (Cayman Chemical Company, Ann Arbor, MI, USA). The correlation between these two methods was good (r=0.5842, p<0.01, n=20). Blood glucose and plasma insulin, corticosterone, total cholesterol, NEFA and triglyceride levels were determined as reported previously [28, 30].
Presentation of results and statistical analysis
Results are the means±SEM for the indicated number of individual mice (in vivo study) or separate experiments (in vitro studies). Unless otherwise indicated, ranges for gene expression levels are presented in each Figure or Table as\(2^{{ - {\left( {\Delta \Delta Ct \pm SEM} \right)}}}\), where SEM is calculated from the ΔΔCt values [21].
Comparisons between two conditions were made using a two-tailed unpaired Student’s t test. Comparisons of at least three conditions were carried out by ordinary ANOVA followed by a Newman–Keuls test. Statistical analysis for gene expression levels was performed on the ΔΔCt values. The correlation analysis was performed using Pearson’s test. Differences were considered statistically significant at p<0.05.
Results
Body weight, food consumption and plasma parameters of obese mice
Body weights and daily food consumption of ob/ob mice were about twofold higher than those of lean mice (Fig. 1a,b). Obese mice were also hyperglycaemic and hyperinsulinaemic as compared with lean mice (Fig. 1c and Table 2) [31]. All these parameters remained rather stable throughout the 3 weeks of the study and were not significantly modified by probucol treatment.
Obese mice were also markedly hypercholesterolaemic as compared with lean mice. As expected from a drug exerting cholesterol-lowering effects [22], probucol fully reversed this abnormality (Table 2). Other plasma lipids did not differ significantly between lean and obese mice, and were not altered by the treatment (Table 2).
Oxidative stress plays a critical role in the pathogenesis of various diseases including diabetes and obesity [18]. Accordingly, obese mice exhibited a two-fold rise in plasma levels of TBARS, a marker of lipid peroxidation and systemic oxidative stress. P, which is mainly a reactive oxygen species (ROS) scavenger, reduced this oxidative stress as demonstrated by the normalisation of plasma TBARS in treated mice (Fig. 1d). Qualitatively comparable results were obtained with plasma measurement of 8-isoprostanes, another marker of lipid peroxidation and oxidative stress (not shown).
Besides type 2 diabetes and leptin deficiency, other endocrine disorders characterise ob/ob mice. As described [28], our obese mice displayed augmented plasma corticosterone levels while their plasma adiponectin concentrations were markedly reduced. Probucol did not correct these abnormalities (Table 2).
Characteristics of obese muscle
Induction of Adipoq expression and adiponectin production in the tibialis anterior
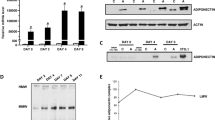
Expression of the gene for adiponectin (Adipoq mRNA levels) was measured by RTQ-PCR in mouse tibialis anterior muscle and compared with the values obtained in subcutaneous (inguinal) fat (Fig. 2a and Table 3). As expected, muscle of lean mice expressed only very low levels of Adipoq mRNA, as compared with fat mice (Table 3). In obese mice, muscle Adipoq mRNA abundance was ∼80-fold higher than in lean littermates and amounted to one-tenth of that detected in fat (Fig. 2a and Table 3, left columns). In contrast to the downregulation observed in visceral fat, Adipoq mRNA was not markedly modified in inguinal fat of obese mice (Table 3), in agreement with a previous report [28]. Probucol treatment of ob/ob mice did not fully restore muscle Adipoq mRNA expression to values measured in lean mice. Nevertheless, Fig. 2a indicates a clear tendency for tibialis anterior Adipoq mRNA levels of probucol-treated mice to be lessened (by about 50%) when compared with untreated obese mice. Muscle Adipoq mRNA levels of the three groups of mice were positively correlated with the two distinct markers of oxidative stress, namely plasma TBARS and 8-isoprostanes (Fig. 2c,d). Because RNA yield (total RNA/mg wet tissue) depends on tissue type (higher in muscle than in fat) and on the ob mutation (dramatically lower in fat of obese or probucol-treated obese mice than in that of lean ones, with no inter-group differences for muscle), Adipoq mRNA data were also expressed per mg tissue. As shown in Table 3 (right columns), 1 mg muscle tissue of obese mice exhibited only two to three times less Adipoq mRNA than 1 mg fat.
Relationships between the level of Adipoq mRNA in tibialis anterior muscle and plasma concentrations of TBARS or 8-isoprostanes. At the end of the study, tibialis anterior muscles and blood were sampled from lean (L, filled squares) mice; untreated ob/ob mice (O, open circles); and ob/ob mice treated with probucol (P, open triangles). TBARS was measured in plasma by a colorimetric method, and 8-isoprostanes by enzyme immunoassay. Adipoq mRNA levels were extracted from whole muscle (a–d) or from laser-microdissected areas of intact myotubes (b), then quantified by RTQ-PCR. Data are presented as relative expression compared with L mice values without (a, b) or with a logarithmic transformation (c, d). Results are normalised to the level of cyclophilin and expressed as means±SEM for ten L, five O and five P mice (a) or two representative L and two representative O mice (b) with ***p<0.001 and *p<0.05 vs L mice. The correlation analysis shown in c and d was performed in all the mice. Dotted lines correspond to the 95% CI for the r value
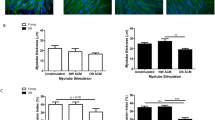
In agreement with mRNA data, immunohistochemistry clearly demonstrated that adiponectin (protein) content was increased in skeletal muscle of obese mice (Fig. 3). Immunostaining of muscle sections showed that adiponectin was localised near to the sarcolemma of muscle fibres in lean mice (Fig. 3a,b) [32]. In obese mice, adiponectin was much more abundantly produced under the sarcolemma, while a faint labelling was also observed in the cytoplasm (Fig. 3c,d). Muscle fibres of obese mice were somewhat disorganised when compared with those of lean mice, a finding confirmed by HES coloration (compare Fig. 4a vs b). Between intact fibres, we found large areas with intense adiponectin staining, which were identified as degenerating myocytes by HES coloration and myosin immunostaining (Fig. 4a–c). The latter labelling was intense in intact myofibres and faint in degenerating cells, in which the typical multinucleated and cylindric morphology of myocytes was preserved. Adiponectin staining was specific as there was no labelling when muscle was incubated with pre-immune serum (Fig. 4e,f) or with an irrelevant anti-thyroglobulin antibody used as control (not shown). A positive control for adiponectin immunostaining obtained from adipose tissue of lean mice (intense cytoplasmic labelling in adipocytes) is also shown in Fig. 4d. Probucol-treated obese mice displayed substantially lower muscle adiponectin labelling than obese mice, a finding consistent with Adipoq mRNA results (Fig. 3e,f).
Immunodetection of adiponectin production in muscle sections of ob/ob mice. Cross (×165, a, c, e) and longitudinal (×165, b, d, f) sections of tibialis anterior muscle sampled from lean (a, b), untreated obese (c, d) or probucol-treated obese mice (e, f). Compared with lean mice, muscle fibres of untreated obese mice showed marked labelling under the sarcolemma and faint cytoplasmic immunoreactivity. A strong labelling was also observed between fibres, which was likely to correspond to degenerating myocytes. Muscle of probucol-treated obese mice was only moderately immunostained. Representative sections are shown
HES and myosin staining in muscle of obese mice and positive and negative controls for adiponectin immunodetection. HES staining of tibialis anterior sections from lean (a, ×40) and obese mice (b, ×103). Immunodetection of myosin in obese mice (c, ×165). Degenerating myotubes indicated by the asterisk display weaker immunostaining when compared with intact ones. d Adiponectin immunostaining in lean mouse adipose tissue sections used as a positive control (cytoplasmic labelling in adipocytes). e, f No adiponectin labelling in muscle incubated with pre-immune serum used as negative controls for lean (e) and obese (f) mice. Representative sections are shown
Adipocytes, if any, were as scarce in muscle sections of obese mice as in those of lean mice. This observation was checked by a morphometric semi-quantitative method of area assessment. The mean relative proportion of myocyte and adipocyte areas was similarly low (<0.01) in tibialis anterior sections of the three groups of mice. This virtual absence of adipocytes allows us to ascertain that our observations did actually originate from Adipoq upregulation in myocytes and did not result from contamination of muscle with adipose tissue. To further support this affirmation, we also measured mRNA levels of Fabp4, an adipose-specific fatty acid transporter. As shown in Table 4, Fabp4 mRNA levels were similar in the three groups of mice. Eventually, we laser microdissected areas of intact myocytes, avoiding all other structures, in sections of two representative lean and obese mice. We then quantified Adipoq mRNA abundance in these samples. As shown in Fig. 2b, Adipoq remained upregulated in myocytes from obese mice. It should be noted that in these two lean and two obese samples, Fabp4 was not detectable (not shown).
Intracellular lipids
Obese mice accumulate lipids in non-adipose tissues such as liver and muscle [33]. We thus looked for ectopic lipid storage in cryocut sections of the tibialis anterior muscle using Oil Red O coloration (Fig. 5a,b). There were almost no lipids in lean muscle. By contrast, in obese mice, lipids were readily detected as intracellular storage in small droplets within myocytes, as well as extracellular deposits. There was no clear difference in ectopic lipids between obese and probucol-treated obese mice (not shown).
Ectopic lipid storage and immunodetection of PPARγ, PRDX3, PRDX5 and caspase-6 in muscle sections of ob/ob mice. At the end of the study, tibialis anterior muscles were sampled from lean or untreated obese mice, then processed for (immuno)histochemistry. Lipids were stained with Oil Red O (ORO, ×165, a, b). Ectopic lipids are stored within and between muscle fibres of obese mice. Immunodetection was performed with specific antibodies against PPARγ (×165, c, d), PRDX3 (×165, e, f), PRDX5 (×165, g, h) and caspase-6 (×103, i, j). When compared with lean mice, immunoreactivity for PPARγ, PRDX3, PRDX5 and caspase-6 was more pronounced in obese mice, with strong nuclear and, in some cases (PPARγ, PRDX), faint cytoplasmic labelling. Representative sections are shown
In this context, we analysed the expression of PPARγ, a transcription factor involved in lipid metabolism and adipocyte differentiation. Pparγ was upregulated in skeletal muscle of obese mice as compared with lean mice (Table 4). This activation was further confirmed by immunohistochemistry. Myotubes of obese mice showed strong labelling for PPARγ (red) (Fig. 5c vs d). Probucol-treated obese mice had intermediate values for these parameters as compared with those of obese and lean mice (Table 4 for mRNAs, and data not shown for immunochemistry).
Oxidative stress and apoptosis
Besides systemic oxidative stress, peripheral tissues of obese mice such as skeletal muscle showed some evidence of local stress as well. Figure 5e–h display the immunohistochemical analysis of PRDX3/5 production. These two peroxidases, which are upregulated during oxidative stress [24], were abundantly present in muscle of obese mice and produced at low levels in those of lean mice (Fig. 5e–h). Again, staining in samples from probucol-treated obese mice was intermediate (not shown). The regulation of PRDX3 and PRDX5 occurred at a translational rather than transcriptional level as confirmed by the lack of mRNA upregulation in obese and probucol-treated obese mice (Table 4).
Moreover, caspase-6, implicated in apoptosis, was activated in myotubes of obese mice, but not in those of lean mice. This activation was confirmed by the intense nuclear labelling visible in Fig. 5j. Thus, the degenerating cells, previously identified by HES staining in muscle of obese mice turned out to be apoptotic, rather than simply necrotic, as either a potential consequence of the pronounced oxidative stress or of another unknown mechanism.
Lastly, the expression of Tnf, which encodes TNF-α, a pro-inflammatory cytokine that has been associated with insulin resistance, oxidative stress and apoptosis, was also slightly upregulated in the tibialis anterior of obese mice as compared with lean mice (Table 4). Again, probucol-treated obese mice showed intermediate values for both Tnf expression (Table 4) and caspase 6 levels (data not shown).
Receptors
Because the major finding of this study was the upregulation of adiponectin, we next investigated whether adiponectin receptors were also regulated in type 2 diabetes. Unlike previously published data [34], we did not find any modification in Adipor1 and Adipor2 mRNA levels in obese and probucol-treated obese mice compared with lean ones (Table 4).
Potential mechanisms underlying adiponectin induction in C2C12 cells
We subsequently attempted to identify the mechanisms underlying muscle adiponectin induction in vitro. Hence, we tested on cultured myotubes the effects of glucose/lipid overload, high insulin and ROS to mimic the in vivo diabetic situation. Besides type 2 diabetes, we also examined the potential repercussions of other endocrine disorders, which are characteristic of ob/ob mice, such as hypercorticosteronaemia and hypoleptinaemia.
We first tested the effects of glucose over a wide range of concentrations (5.5–25 mmol/l) and we more specifically addressed the repercussions of glucotoxicity (using glucosamine or AGE) (Table 5). These experimental conditions did not affect Adipoq mRNA levels in C2C12 myotubes. Similarly, insulin, dexamethasone, leptin or cholesterol were without effect (Table 5).
We next examined the potential role of lipotoxicity/ectopic lipid accumulation and oxidative stress on adiponectin induction. To this end, we cultured C2C12 cells with a mixture of triglycerides (Structolipid) for 48 h. In those conditions, Adipoq mRNA levels rose by ∼225%. This effect was almost totally reversed when the antioxidant, NAC, was added to the medium (Fig. 6a). These results suggest a role of ectopic muscular lipids in the adiponectin upregulation observed in the tibialis anterior of ob/ob mice.
Influence of lipids and oxidative stress on Adipoq mRNA levels in C2C12 myotubes. a C2C12 cells were cultured for 48 h in the absence (control, CTRL) or in the presence of Structolipid (0.2 % v/v; Lipids) and/or N-acetylcysteine (NAC; 10 mmol/l). b, c C2C12 cells were cultured for 18 h (b) or 8 h (c) in the absence (control, CTRL) or in the presence of 100 μmol/l H2O2 (b) or 100 μmol/l 4-hydroxy-2-nonenal (HNE; c) and/or NAC (10 mmol/l). The antioxidant was added 30 min before the other products. mRNA levels were quantified by RTQ-PCR and are presented as relative expression compared with control values. Results are normalised to the level of cyclophilin and expressed as means±SEM for six independent experiments. ### p<0.001 vs CTRL; + p<0.05 vs Lipids; ***p<0.001 vs all conditions
To investigate the contribution of oxidative stress we cultured C2C12 myotubes with ROS producers, such as H2O2 or the active end-product of lipid peroxidation, HNE. When compared with control values, both conditions increased levels of Adipoq mRNA (∼2.6-fold and ∼7-fold, respectively). Again, NAC abrogated this effect of the ROS (Fig. 6b,c).
All these in vitro data provide evidence for a role of increased oxidative stress and ROS production in adiponectin upregulation. In this context, we measured the production of adiponectin protein in homogenates of C2C12 cells incubated with ROS producers, HNE and H2O2 (Fig. 7a,b). Adiponectin levels rose in both conditions (by 200 and 150%, respectively).
Influence of oxidative stress on adiponectin levels in C2C12 myotubes. C2C12 cells were cultured for 8 h (a) or 18 h (b) in the absence (control, CTRL) or in the presence of 100 μmol/l 4-hydroxy-2-nonenal (HNE; a) or 100 μmol/l H2O2 (b). Adiponectin levels were measured in cell homogenates by RIA and expressed as nanogram per milligram protein. Results are the means±SEM for six independent experiments. **p<0.01 and *p<0.05 vs CTRL
Lastly, we measured Pparγ mRNA levels in all the experiments described in Fig. 5. Concurrently with the upregulation of Adipoq expression, levels of Pparγ mRNA, present at low levels in C2C12 myotubes, were increased by two- to four-fold in the presence of Structolipid, H2O2 or HNE (p≤0.05 for each condition). This increase was again prevented by the addition of NAC (not shown).
Discussion
In this study, we showed that Adipoq expression and adiponectin production were paradoxically upregulated in the skeletal muscle of obese and diabetic ob/ob mice, while they decreased in some adipose tissue sites [28], with a decrease in circulating levels as a consequence. This upregulation of adiponectin did occur in myocytes, as shown by laser microdissection experiments, and was associated with ectopic lipid storage, markers of oxidative stress and apoptosis.
The cellular mechanisms responsible for adiponectin upregulation were further characterised in cultured murine myotubes. We attempted to isolate each pathophysiological feature of the ob/ob mice that could trigger muscle adiponectin induction and then evaluated their repercussions in vitro. Several conditions were tested. Firstly, glucotoxicity. Culturing myotubes in the presence of high glucose, glucosamine or AGE did not result in any significant changes in Adipoq mRNA. Secondly, hyperinsulinaemia/insulin resistance and other endocrine disorders such as leptin deficiency or hypercorticosteronaemia. These potential contributors were excluded, as insulin, leptin or dexamethasone did not affect Adipoq mRNA. Thirdly, hyperlipidaemia/lipid overload and oxidative stress. Although the role of cholesterol was ruled out, a mixture of triglycerides added to the medium induced Adipoq expression. Likewise, inducers of ROS, such as H2O2 and HNE, upregulated adiponectin. Thus, ectopic lipid storage may lead to high amounts of lipoperoxidation end-products, which may in turn produce ROS, thereby inducing adiponectin in muscle. This hypothesis was supported by in vivo data. Obese muscle exhibited both lipid infiltration and signs of oxidative stress. Moreover, muscle Adipoq mRNA did positively correlate with plasma TBARS and 8-isoprostanes. Eventually, treatment of the obese mice with the ROS scavenger probucol led to a decrease in plasma and muscle oxidative stress markers, together with a partial restoration of Adipoq mRNA levels and a clear-cut diminution of the adiponectin protein content in muscle. We thus extended the recent concept that lipid accumulation and oxidative stress in adipose tissue deregulate adipokine production to non-adipose tissues [18]. Fourthly, pro-inflammatory cytokines. Diabetes and obesity are now commonly considered as chronic inflammatory states. Plasma concentrations of inflammatory adipokines, such as TNF-α and IL-6, are increased in those pathological situations [35]. We showed that Tnf was slightly upregulated in the tibialis anterior of obese mice. Because adiponectin could be induced in mouse skeletal muscle and in cultured myotubes in response to LPS or pro-inflammatory cytokines [21], increased TNF-α may further amplify the rise in muscle adiponectin levels in obesity.
The molecular mechanisms underlying adiponectin upregulation in muscle occurred at the pre-translational level. Along with Adipoq mRNA, Pparγ was upregulated in the skeletal muscle of ob/ob mice and its expression was also induced in every condition that stimulated adiponectin in C2C12 myotubes. It is well established that in the adipocyte, PPARγ stimulates the transcriptional activity of the gene encoding adiponectin through a PPARγ-responsive element in the promoter [36]. Whether increased production of PPARγ also stimulates adiponectin production in muscle remains to be established. In contrast to the upregulation of adiponectin and PPARγ found in muscle (this study), oxidative stress downregulated their expression in the adipocyte [18]. Likewise, LPS or pro-inflammatory cytokines upregulated adiponectin production in muscle or myotubes, while inhibiting its production in adipocytes [21, 37]. Thus, in many respects, adiponectin regulation in muscle seems to be fully distinct, if not opposite to that described in adipose tissue.
Although the bulk of adiponectin originates from fat, we estimated, based our own and other data (Table 3, right columns; [38–41]), that as much as 1/10th to 1/15th of systemic adiponectin levels might be accounted for by muscle production in obese mice, while this proportion fell to ∼1/175th in lean ones. Yet, the systemic contribution of muscle in obese mice is still insufficient to reverse the hypoadiponectinaemia. Nevertheless, this muscular production of adiponectin in obesity may be of physiological significance because of its local action. Firstly, it is worth noting that Adipoq mRNA concentration in obese muscle was rather close to that found in the same amount of fat (Table 3). Secondly, the local efficacy of adiponectin on myocytes might be high since it is directly acting on these cells via autocrine or paracrine mechanisms [42]. Thirdly, this potency could even be further enhanced by post-translational modifications of the adipokine (e.g. a proteolytic cleavage leading to its globular form) [4]. In consideration of the local action of adiponectin, it should be stressed that, at variance with another study performed in a very small number of ob/ob mice [34], the expression of Adipor1 and Adipor2 was not modified in the muscle of our obese mice. Our data are in agreement with the unaltered expression of adiponectin receptors in muscle of type 2 diabetic patients [43]. The lack of downregulation of these receptors indicates that the potency of locally produced adiponectin may be preserved.
The physiological relevance of this upregulation of adiponectin in diabetic muscle is still under investigation. On the one hand, adiponectin exerts anti-inflammatory properties on several cell types or tissues and suppresses superoxide generation in endothelial cells [10–12, 14]. As already suggested, induction of adiponectin in muscle could be viewed as a protective mechanism against deleterious inflammatory reactions and oxidative stress. On the other hand, adiponectin upregulation in diabetic muscle could also be a useful means to counteract insulin resistance as well as ectopic lipid deposit [44]. This may be achieved thanks to its ability to stimulate fatty acid oxidation and glucose transport in skeletal muscle [2, 3, 7].
In conclusion, adiponectin is upregulated in muscle of obese and diabetic ob/ob mice. This induction may result from lipotoxicity and related oxidative stress. This finding could be viewed as a local protective mechanism to counteract oxidative damage, ectopic lipid storage and insulin resistance.
Abbreviations
- Adipor:
-
adiponectin receptor
- HES:
-
haematoxylin–eosin–safran
- HNE:
-
4-hydroxy-2-nonenal
- LPS:
-
lipopolysaccharide
- NAC:
-
N-acetylcysteine
- PPARγ:
-
peroxisome proliferator-activated receptor γ
- PRDX3/5:
-
peroxiredoxin 3/5
- ROS:
-
reactive oxygen species
- RTQ-PCR:
-
real-time quantitative PCR
- TBARS:
-
thiobarbituric acid reactive substance
References
Lyon CJ, Law RE, Hsueh WA (2003) Minireview: adiposity, inflammation, and atherogenesis. Endocrinology 144:2195–2200
Fruebis J, Tsao TS, Javorschi S et al (2001) Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98:2005–2010
Yamauchi T, Kamon J, Minokoshi Y et al (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295
Yamauchi T, Kamon J, Ito Y et al (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953
Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L (2001) Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest 108:1875–1881
Tomas E, Tsao TS, Saha AK et al (2002) Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99:16309–16313
Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ (2003) The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112:91–100
Guerre-Millo M (2004) Adipose tissue and adipokines: for better or worse. Diabetes Metab 30:13–19
Ouchi N, Kihara S, Arita Y et al (1999) Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100:2473–2476
Ouchi N, Kihara S, Arita Y et al (2000) Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 102:1296–1301
Yokota T, Oritani K, Takahashi I et al (2000) Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96:1723–1732
Nakanishi S, Yamane K, Kamei N, Nojima H, Okubo M, Kohno N (2005) A protective effect of adiponectin against oxidative stress in Japanese Americans: the association between adiponectin or leptin and urinary isoprostane. Metabolism 54:194–199
Motoshima H, Wu X, Mahadev K, Goldstein BJ (2004) Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun 315:264–271
Cnop M, Havel PJ, Utzschneider KM et al (2003) Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46:459–469
Arita Y, Kihara S, Ouchi N et al (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83
Hotta K, Funahashi T, Arita Y et al (2000) Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20:1595–1599
Furukawa S, Fujita T, Shimabukuro M et al (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761
Ryo M, Nakamura T, Kihara S et al (2004) Adiponectin as a biomarker of the metabolic syndrome. Circ J 68:975–981
Havel PJ (2002) Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol 13:51–59
Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM (2004) Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology 145:5589–5597
Benson GM, Schiffelers R, Nicols C et al (1998) Effect of probucol on serum lipids, atherosclerosis and toxicology in fat-fed LDL receptor deficient mice. Atherosclerosis 141:237–247
Gerard AC, Many MC, Daumerie C et al (2002) Structural changes in the angiofollicular units between active and hypofunctioning follicles align with differences in the epithelial expression of newly discovered proteins involved in iodine transport and organification. J Clin Endocrinol Metab 87:1291–1299
Leyens G, Donnay I, Knoops B (2003) Cloning of bovine peroxiredoxins—gene expression in bovine tissues and amino acid sequence comparison with rat, mouse and primate peroxiredoxins. Comp Biochem Physiol B Biochem Mol Biol 136:943–955
Weibel ER (1981) Stereological methods in cell biology: where are we—where are we going? J Histochem Cytochem 29:1043–1052
Anon (1968) Methods for fats and lipids. In: Luna LG (ed) Manual of histologic staining methods of the Armed Forces Institute of Pathology. 3rd edn. McGraw-Hill, New York, pp 140–142
Uchida Y, Ohba K, Yoshioka T, Irie K, Muraki T, Maru Y (2004) Cellular carbonyl stress enhances the expression of plasminogen activator inhibitor-1 in rat white adipocytes via reactive oxygen species-dependent pathway. J Biol Chem 279:4075–4083
Delporte ML, El Mkadem SA, Quisquater M, Brichard SM (2004) Leptin treatment markedly increased plasma adiponectin but barely decreased plasma resistin of ob/ob mice. Am J Physiol Endocrinol Metab 287:E446–E453
Scoccia AE, Molinuevo MS, McCarthy AD, Cortizo AM (2001) A simple method to assess the oxidative susceptibility of low density lipoproteins. BMC Clin Pathol 1:1
Cani P, Neyrinck A, Maton N, Delzenne N (2005) Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res 13:1000–1007
Bailey CJ, Flatt PR (1987) Increased responsiveness to glucoregulatory effect of opiates in obese-diabetic ob/ob mice. Diabetologia 30:33–37
Punyadeera C, Zorenc AH, Koopman R et al (2005) The effects of exercise and adipose tissue lipolysis on plasma adiponectin concentration and adiponectin receptor expression in human skeletal muscle. Eur J Endocrinol 152:427–436
Ide T, Tsunoda M, Mochizuki T, Murakami K (2004) Enhancement of insulin signaling through inhibition of tissue lipid accumulation by activation of peroxisome proliferator-activated receptor (PPAR) alpha in obese mice. Med Sci Monit 10:BR388–BR395
Tsuchida A, Yamauchi T, Ito Y et al (2004) Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem 279:30817–30822
Lacquemant C, Vasseur F, Lepretre F, Froguel P (2003) [Adipocytokins, obesity and development of type 2 diabetes]. Med Sci (Paris) 19:809–817
Iwaki M, Matsuda M, Maeda N et al (2003) Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52:1655–1663
Maeda N, Takahashi M, Funahashi T et al (2001) PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50:2094–2099
Ferre P, Leturque A, Burnol AF, Penicaud L, Girard J (1985) A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J 228:103–110
Trostler N, Romsos DR, Bergen WG, Leveille GA (1979) Skeletal muscle accretion and turnover in lean and obese (ob/ob) mice. Metabolism 28:928–933
Breslow MJ, Min-Lee K, Brown DR, Chacko VP, Palmer D, Berkowitz DE (1999) Effect of leptin deficiency on metabolic rate in ob/ob mice. Am J Physiol 276:E443–E449
Harris RB, Hausman DB, Bartness TJ (2002) Compensation for partial lipectomy in mice with genetic alterations of leptin and its receptor subtypes. Am J Physiol Regul Integr Comp Physiol 283:R1094–R1103
Staiger H, Kausch C, Guirguis A et al (2003) Induction of adiponectin gene expression in human myotubes by an adiponectin-containing HEK293 cell culture supernatant. Diabetologia 46:956–960
Debard C, Laville M, Berbe V et al (2004) Expression of key genes of fatty acid oxidation, including adiponectin receptors, in skeletal muscle of Type 2 diabetic patients. Diabetologia 47:917–925
Rakatzi I, Mueller H, Ritzeler O, Tennagels N, Eckel J (2004) Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia 47:249–258
Acknowledgements
We are grateful to A. M. Pottier, I. Bauche and J. Jortay for skilful assistance. This work was supported by grants from the Foundation of Scientific and Medical Research (3.4580.05), from the Fonds National de la Recherche Scientifique (FNRS; 1.5.189.04) and grant ARC 05/10-328 from the General Division of Scientific Research. A. M. Delaigle is Research Fellow of the FNRS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delaigle, A.M., Senou, M., Guiot, Y. et al. Induction of adiponectin in skeletal muscle of type 2 diabetic mice: in vivo and in vitro studies. Diabetologia 49, 1311–1323 (2006). https://doi.org/10.1007/s00125-006-0210-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0210-y