Abstract
Background
Diabetes is common in dogs, with an estimated prevalence of 0.32% in the UK. Clinical signs, as in man, include polydipsia, polyuria and weight loss, associated with hyperglycaemia and glucosuria. Diabetes typically occurs in dogs between 5 and 12 years of age, and is uncommon under 3 years of age. Breeds predisposed to diabetes include the Samoyed, Tibetan Terrier and Cairn Terrier, while others such as the Boxer and German Shepherd Dog seem less susceptible. These breed differences suggest a genetic component, and at least one dog leucocyte antigen haplotype (DLA DRB1*009, DQA1*001, DQB1*008) appears to be associated with susceptibility to diabetes.
Methods
Canine diabetes can be classified into insulin deficiency diabetes (IDD), resulting from a congenital deficiency or acquired loss of pancreatic beta cells, or insulin resistance diabetes resulting mainly from hormonal antagonism of insulin function.
Results
There is no evidence for a canine equivalent of human type 2 diabetes. Adult-onset IDD, requiring insulin therapy, is the most common form, with pancreatitis and/or immune-mediated beta cell destruction considered to be the major underlying causes of the disease.
Discussion
Autoantibodies to insulin, recombinant canine GAD65 and/or canine islet antigen-2 have been identified in a proportion of newly diagnosed diabetic dogs, suggesting that autoimmunity is involved in the pathogenesis of disease in some patients.
Conclusion
The late onset and slow progression of beta cell dysfunction in canine diabetes resembles latent autoimmune diabetes of the adult in man.
Similar content being viewed by others
Introduction
Historically, dogs have played a pivotal role in our understanding of the pathophysiology and treatment of diabetes mellitus. In 1889, Joseph von Mering and Oskar Minkowski discovered that removing the pancreas from healthy dogs resulted in polyuria and polydipsia. They realised that they had created an animal model of diabetes, and correctly concluded that the pancreas must secrete an ‘anti-diabetogenic factor’ (later found to be insulin) that enables the body to utilise glucose [1]. In 1921, a diabetic dog became the first recipient of insulin therapy [2], paving the way for the treatment of human patients. Diabetic Beagle dogs (in whom diabetes is usually induced by pancreatectomy) are still used for research, particularly in islet cell transplantation studies [3], although rodents, such as the non-obese diabetic mouse and BB rat, have long replaced the dog as the major animal models of diabetes.
It may surprise some clinicians and researchers to learn that pet dogs suffer from spontaneous diabetes and are diagnosed and treated by veterinary surgeons in much the same way as humans. Research into the pathogenesis of canine diabetes has, unfortunately, failed to keep pace with its human counterpart, particularly when it comes to understanding the genetic and immunological basis of the disease. This review will highlight the major findings of a 3-year (December 2000 to December 2003) multicentre study into diabetes in the UK dog population. This collaborative project, involving all the UK veterinary schools, led to the formation of a national canine diabetes register, a centralised database containing clinical, biochemical, serological and genetic information from cases recruited in over 100 first-opinion veterinary practices. Since the canine population contains both purebred as well as outbred animals, and diabetic dogs share the same environment as humans, we may perhaps be able to learn something of benefit from our closest companions.
Clinical features of canine diabetes
Diabetes mellitus is one of the commonest endocrine disorders in dogs, and presents, as in man, with polydipsia, polyuria, polyphagia and weight loss. The prevalence of canine diabetes has been estimated to be anywhere between 0.0005% and 1.5% [4, 5]. We analysed a database of insured pet dogs in the UK (n=46 593) and found that 0.32% (n=151) had diabetes (data courtesy of Pet Protect Insurance, London, UK). The proportion of diabetic dogs referred to second-opinion veterinary hospitals in North America increased from 19 per 10,000 to 64 per 10,000 over the past 30 years [6], suggesting that the incidence of diabetes has increased in dogs as in man. Alternatively, this increase might be explained by improvements in the diagnosis and management of diabetes by first-opinion veterinary practitioners and a greater willingness to refer more difficult cases.
Diabetes typically occurs in dogs between 5 and 12 years of age [7]. In our database the median age at diagnosis was 9 years (range 3 months to 18 years), illustrating that canine diabetes is generally a disease of middle-aged and older dogs (Fig. 1). Juvenile‐onset diabetes is uncommon in dogs, and in our series of 500 affected animals, only nine were less than 12 months of age.
Certain breeds of dog appear to be predisposed to diabetes. A database containing medical records of over 6,000 diabetic dogs from 24 veterinary schools in North America identified breeds including the Miniature Schnauzer, Bichon Frise, Miniature Poodle, Samoyed and Cairn Terrier as having an increased risk of the disease [6]. A similar breed distribution was seen in our own database (Table 1), in which the Samoyed, Tibetan Terrier and Cairn Terrier had the highest relative risk of diabetes. Some popular breeds, including the Boxer, German Shepherd Dog and Golden Retriever, appear to have a reduced risk of diabetes [6–8]. These breed differences strongly suggest a genetic component to disease, although this area of research has received little attention. Preliminary results from our collaborative study suggest that MHC genes are associated with diabetes susceptibility in dogs, as discussed later [9].
Previous surveys showed that female dogs were more likely to develop diabetes, and represented around 70% of cases [7, 10]. However, this bias is less apparent in our current database in which 53% are female. Diabetes in sexually intact female dogs is often associated with the progesterone-dominated phase of dioestrus and, as will be seen, resembles gestational diabetes in humans. Many veterinary surgeons in the UK now recommend elective neutering before 12 months of age, and this, together with a simultaneous reduction in the use of synthetic progestagens, is likely to explain the decline of dioestrous diabetes in female dogs.
We found a seasonal pattern in the diagnosis of canine diabetes [8]; twice as many dogs were diagnosed between November and January as between July and September (Fig. 2). A similar pattern has previously been reported in the USA [11], and is also seen in human type 1 diabetes [12]. It is tempting to speculate that the similar seasonality of onset of diabetes seen in dog and man is associated with common environmental factors.
Diagnosis and management of diabetes in dogs
Diabetes is diagnosed in dogs on the basis of clinical history, persistent hyperglycaemia (>9 mmol/l) and glucosuria. The initial signs of diabetes can go unnoticed, with the result that dogs sometimes present in diabetic ketoacidosis with anorexia, lethargy, vomiting and dehydration. This can be life threatening without appropriate therapy. Veterinary practitioners often treat diabetes without performing ancillary diagnostic tests, but the recent development of RIAs for canine insulin and C-peptide should mean that diabetic dogs will, in future, undergo additional assessment of pancreatic beta cell function at diagnosis. Other useful biochemical markers include serum trypsin-like immunoreactivity and canine pancreatic lipase immunoreactivity, which are both indicative of exocrine pancreatic disease. Further endocrine testing (e.g. adrenocorticotrophin stimulation) is sometimes required if concurrent hyperadrenocorticism is suspected.
Most diabetic dogs suffer from insulin deficiency and require exogenous insulin. Oral hypoglycaemic drugs are not useful. Most dogs are injected once daily, although twice-daily administration can lead to better glycaemic control and reduced risk of insulin-induced hypoglycaemia [13].
The introduction of diagnostic testing of blood samples for fructosamine and glycosylated haemoglobin has provided additional options for monitoring of glycaemic control in veterinary patients. We have evaluated an HbA1c meter designed for human diabetic blood samples for use in diabetic dogs (Haemaquant/Glycosal; Provalis Diagnostics, Deeside, Flintshire, Wales, UK). In normoglycaemic dogs the range for HbA1c was 2.5–3.7%, as against 2.5–7.0% in animals with diabetes [14]. We generally take a value below 5.0% to represent good glycaemic control.
Although insulin therapy can be used successfully in the majority of cases, hypoglycaemic seizures, cataract formation or ketoacidosis may develop. Whereas diabetic dogs rarely suffer from nephropathy or peripheral neuropathy, diabetes is considered a risk factor for development of atherosclerosis, although this is usually not of clinical relevance and only comes to light at post mortem [15].
Pathology of canine diabetes
Typical histopathological findings in the human pancreas at the onset of type 1 diabetes include reduced beta cell numbers and lymphocytic infiltration of pancreatic islets [16]. Histopathological studies of the pancreas in dogs are few and far between, and mostly describe pathology in dogs with congenital diabetes or in treated diabetic dogs following euthanasia. Gepts and Toussaint [17] reported a heterogeneous pathology, but noted some similarities with human type 1 diabetes. A later study described insulitis in pancreatic biopsies in six of 18 diabetic dogs, and generalised pancreatic inflammation was seen in a further five [18]. Studies in dogs with congenital diabetes show a reduction in the number and size of islets and hydropic degeneration/vacuolation of beta cells, consistent with beta cell aplasia, hypoplasia or abiotrophy [19, 20]. It is clear that canine diabetes is heterogeneous, and that beta cell dysfunction may arise for differing reasons.
Classification of canine diabetes
There are no internationally accepted criteria for the classification of canine diabetes. A UK-based canine diabetes interest group has been formed in an attempt to standardise diagnostic criteria for the different types of diabetes in dogs and to promote a collaborative research effort between veterinary schools and veterinary diagnostic laboratories. It is hoped that improved phenotyping at diagnosis will allow disease variants to be more clearly defined and will permit a more focused approach to their investigation.
The classification developed for human diabetes is not easy to apply to dogs. Previously, canine diabetes has been classified as either insulin dependent or non-insulin dependent, although virtually all diabetic dogs require insulin therapy. At the Royal Veterinary College we currently use a classification system for canine diabetes which is based on disease pathogenesis rather than the clinical response to insulin therapy.
Classification of canine diabetes mellitus Insulin deficiency diabetes (IDD) Primary IDD in dogs is characterised by a progressive loss of pancreatic beta cells. The aetiology of beta cell deficiency/destruction in diabetic dogs is currently unknown but a number of diseases processes are thought to be involved: • Congenital beta cell hypoplasia/abiotrophy • Beta cell loss associated with exocrine pancreatic disease • Immune-mediated beta cell destruction • Idiopathic Insulin resistance diabetes (IRD) Primary IRD usually results from antagonism of insulin function by other hormones • Dioestrous/gestational diabetes • Secondary to other endocrine disorders • Hyperadrenocorticism • Acromegaly • Iatrogenic • Synthetic glucocorticoids • Synthetic progestagens • Glucose intolerance associated with obesity might contribute to insulin resistance but is not a primary cause of diabetes in dogs |
Canine diabetes can, therefore, be broadly divided into two groups; primary insulin deficiency diabetes (IDD), in which hyperglycaemia is due to hypoinsulinaemia, and primary insulin resistance diabetes (IRD), in which hyperglycaemia co-exists with hyperinsulinaemia.
Chronic hyperglycaemia (i.e. blood glucose >14 mmol/l) can in itself produce permanent beta cell dysfunction in dogs [21]. Since there is often a delay between the onset of diabetes and the owner seeking veterinary attention, dogs with primary IRD often progress to IDD, presumably as the result of secondary loss of beta cells associated with uncorrected hyperglycaemia. Such cases may appear to have IDD at diagnosis, but glycaemic control may prove difficult until the underlying cause of insulin resistance has been addressed.
Insulin deficiency diabetes
Congenital beta cell aplasia/abiotrophy
There are rare reports of diabetes in dogs less than 12 months of age. In one study of four such dogs, the histopathology was consistent with congenital beta cell aplasia [19]. An inherited, early-onset form of diabetes characterised by atrophy of pancreatic beta cells has also been reported in Keeshond dogs [22]. Mating studies demonstrated that the condition was probably due to an autosomal recessive mutation, although the precise genetic defect has not been elucidated [23]. We identified six Labrador Retrievers (three dogs from the same litter) that developed clinical signs of diabetes at around 12 weeks of age, also suggesting a congenital beta cell abnormality. Histopathological examination of a pancreas biopsy from one of these dogs demonstrated marked islet hypoplasia (thanks to J. C. Patterson-Kane, Royal Veterinary College). Blood samples and DNA have been archived, but no genetic analysis has been performed to date.
Exocrine pancreatic disease and canine diabetes
Acute pancreatitis in dogs is characterised by abdominal pain and vomiting, and can progress to life-threatening electrolyte disturbances, dehydration and disseminated intravascular coagulation [24]. Dogs with chronic pancreatitis tend to present with subtle, non-specific clinical signs and can be difficult to diagnose [25]. Although pancreatitis primarily affects exocrine tissue, the inflammatory response may affect endocrine function, and beta cells, in particular, seem to be sensitive to the deleterious effects of inflammatory mediators including IL-1β and TNF-α [26]. It has been proposed that pancreatitis in dogs might initiate beta cell damage, and that the subsequent release of antigens might stimulate an immune response that could potentially exacerbate islet destruction [27].
Biochemical or histopathological evidence of pancreatitis has been reported in 28–40% of diabetic dogs [18, 28], and it is possible that clinical signs of diabetes could be the first indication of subclinical, low-grade exocrine pancreatic disease. Preliminary findings indicate that dogs confirmed as suffering from chronic pancreatitis can have impaired glucose tolerance, identified by glucagon-stimulation testing (P. Watson, University of Cambridge, personal communication), although it is still unclear what proportion progress to overt diabetes.
Chronic pancreatitis can lead to exocrine pancreatic insufficiency in both humans and dogs, and gives rise to diabetes in a proportion of animals [25]. The most common form of exocrine pancreatic insufficiency in dogs results from pancreatic acinar atrophy [29], most commonly seen in German Shepherd Dogs, which represent up to 66% of all cases of pancreatic insufficiency [30]. A marked T-lymphocyte infiltration of the exocrine tissue is seen in pancreatic acinar atrophy, suggesting an immune-mediated component. Endocrine tissue is, however, usually preserved in such cases and diabetes, as previously noted, is uncommon in this breed.
The Miniature Schnauzer has a high risk of diabetes and is also predisposed to idiopathic hyperlipoproteinaemia, which is characterised by an increased plasma triglyceride concentration and excessive circulating VLDL [31]. Since hyperlipidaemia is believed to be a predisposing factor for pancreatitis, it has been proposed that dogs with idiopathic hyperlipoproteinaemia may develop diabetes as a consequence of repeated attacks of pancreatic inflammation [24].
In our own study, eight of 253 diabetic dogs had clinical signs and biochemical abnormalities consistent with pancreatitis, and a further seven had concurrent exocrine pancreatic insufficiency [8]. This probably underestimates the proportion with subclinical exocrine pancreatic disease, given the poor sensitivity and specificity of the diagnostic tests available [32]. Serum biochemical markers such as amylase and lipase are commonly, but not invariably, raised in the presence of pancreatic inflammation [33]. Serum amylase can be elevated in canine diabetes, although this is not a consistent finding [34]. Canine trypsin-like immunoreactivity can be a useful marker of acute pancreatitis, but is usually low in end-stage disease [35]. Canine pancreatic lipase immunoreactivity may prove a more sensitive marker of pancreatic inflammation in dogs [36]. In our study, values above the upper limit of the normal reference range (102.1 μg/l) were found in five of the 30 dogs tested, but none of those with diabetes had levels diagnostic for pancreatitis (>200 μg/l) [37]. Serial measurement of serum pancreatic lipase immunoreactivity suggests that subclinical pancreatic inflammation is relatively common in dogs with diabetes (unpublished data). However, it is not clear whether pancreatitis is the primary disease, increasing the risk of development of diabetes, or vice versa [38]. Further work is required to clarify the association between pancreatitis and diabetes in dogs.
Autoimmunity and canine diabetes
Human type 1 diabetes results from autoimmune destruction of pancreatic beta cells. Immune-mediated beta cell damage may also be involved in the pathogenesis of canine diabetes [39], although the evidence is less convincing than in humans and rodents. As we have seen, lymphocytic infiltration of pancreatic islets is seen in only a proportion of dogs with adult-onset diabetes [18], and is not a feature of dogs with juvenile-onset disease [20].
Most diabetic dogs are middle aged and older when clinical signs become apparent. The rate of progression to absolute insulin deficiency has not been studied extensively, although C-peptide values in the newly diagnosed are higher than in longer duration animals [40]. Canine diabetes might therefore be comparable to the latent autoimmune diabetes of the adult form of type 1 diabetes in man [41], which is characterised by slowly progressive beta cell destruction [42].
There are, to date, no published studies of cell-mediated immune responses in canine diabetes. Anti-islet cell antibodies have been documented in 50% of newly diagnosed diabetic dogs, suggesting that autoantibodies are present in canine diabetes [43] and that immune-mediated beta cell destruction may be a factor in some animals [44]. Diabetic serum also contains antibodies capable of stimulating complement-mediated beta cell lysis [39], illustrating that these autoantibodies might be involved in the pathogenesis of disease.
The antigen specificity of such anti-islet immune responses is currently unknown. Insulin did not appear to be the primary target in preliminary studies of canine anti-beta cell reactivity, although anti-insulin antibodies have been documented in newly diagnosed dogs [27]. In our study, anti-insulin antibodies were detected by ELISA in five of 40 dogs with newly diagnosed diabetes [37], although this is not a particularly sensitive method, with relatively high background values in non-diabetic control dogs. We have also screened diabetic sera against recombinant canine proinsulin by western blotting, and observed proinsulin reactivity in six of 15 newly diagnosed cases (unpublished data). We hope to be able to screen diabetic sera using a liquid-phase RIA against canine insulin in the near future.
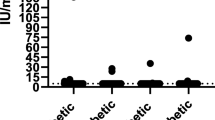
Work by our group has provided some evidence for the presence of GAD65 and islet antigen-2 (IA-2) autoantibodies in diabetic dogs, suggesting that these antigens might be involved in the autoimmune response in canine diabetes. We have cloned and expressed recombinant canine GAD65 (GenBank Accession Number DQ 060442) and the C-terminal region of canine IA-2 (IA-2/Cterm: amino acids 771–979) [45]. In collaboration with S. Weenink and M. Christie at King’s College London, we have adapted the human GAD65 and IA-2 radio-immunoprecipitation assay to screen canine sera against these recombinant canine beta cell proteins. Human GAD65 antibody-positive diabetic sera and monoclonal antibodies raised against human GAD65 also recognised canine GAD65 and were used as positive controls. In a preliminary study, six of 30 newly diagnosed diabetic dogs demonstrated anti-GAD65 reactivity (Fig. 3). Five of the 30 diabetic dogs demonstrated reactivity to IA-2/Cterm, with two of these dogs also reacting to GAD65.
A proportion of diabetic dogs show anti-GAD65 reactivity. Sera from newly diagnosed diabetic dogs (n=30) and normoglycaemic dogs (n=25) were incubated with [35S]methionine-labelled recombinant canine GAD65. Serum reactivity was measured by immunoprecipitation using Protein A-Sepharose. Each data point represents the mean of triplicates for each dog. GAD65 antibody‐positive (red square) and ‐negative (green circle) human sera were used as controls. The 95% CI (mean+2×SD cpm normoglycaemic dogs) is indicated by the horizontal dotted line
No exclusion criteria were applied to the diabetic dogs included in these pilot autoantibody screening experiments and this initial cohort is likely to contain animals suffering from other, non-immune-mediated, forms of IDD and IRD. Indeed, of the autoantibody-negative dogs, three female dogs were likely to be suffering from dioestrous diabetes, four dogs had evidence of pancreatitis and three dogs had congenital disease. This preliminary work suggests that a proportion of dogs, in which no other underlying cause can be identified, might be experiencing immune-mediated beta cell destruction. Further work is planned to characterise the autoantibody profiles in dogs with suspected immune-mediated diabetes.
Genetic susceptibility to type 1 diabetes in man has a strong association with the genes encoding MHC class II [46]. Susceptibility is linked to HLA DQA and DQB genes, influenced by the DR genes [47]. The observation that certain breeds of dog are predisposed to diabetes suggests that there is also a genetic component to canine diabetes. If beta cell loss occurs via an immune-mediated process, one might expect susceptibility to be associated with the dog leucocyte antigen (DLA) genes, encoding the canine MHC. In collaboration with Dr Lorna Kennedy and Professor Bill Ollier at the University of Manchester, we DLA genotyped 122 dogs with diabetes. One haplotype (DLA DRB1*009, DQA1*001, DQB1*008) was over-represented as compared with breed-matched controls with an odds ratio of 3.31 (95% CI 1.24–9.16) [9]. In addition, DLA DQA1 alleles coding for arginine at position 55 (Arg55) in hypervariable region 2 were associated with diabetes (Fig. 4) and might be equivalent to the association with HLA DQA1 Arg52 in human diabetes [48]. It is interesting to note that the ‘increased-risk’ haplotype is common in Samoyed dogs, the breed at greatest risk of developing diabetes. Additionally, two dogs (one cross-breed and one Cavalier King Charles Spaniel) were suffering from concurrent diabetes and Addison’s disease, which is likely to be equivalent to human autoimmune polyendocrine syndrome type II and both dogs had the ‘increased-risk’ DLA haplotype. The samples we used for immunogenetic analysis were drawn from a heterogeneous population of diabetic dogs. More effective phenotyping should, in time, improve our understanding of the genetic factors associated with the different forms of the disease.
Susceptibility to canine diabetes is associated with DLA genes. In a preliminary study of 122 diabetic dogs, one haplotype (DRB1*009/DQA1*001/DQB1*008) was over-represented compared with breed-matched controls. Additionally, DQA1 alleles coding for arginine at position 55 (Arg55) in hypervariable region 2 were associated with diabetes. (Reproduced with thanks to L. J. Kennedy, A. Barnes, D. Isherwood and W. E. R. Ollier, Centre for Integrated Genomic Medical Research, University of Manchester, UK)
Insulin resistance diabetes
Dioestrous diabetes in female dogs
The most common form of IRD occurs during the dioestrous phase of the reproductive cycle of female dogs, which resembles gestational diabetes in humans. Dogs are non-seasonally mono-oestrus and following a season (oestrus), all bitches enter a progesterone-dominated luteal phase (dioestrus) that lasts around 60 days. Progesterone acts on the canine mammary gland stimulating production of growth hormone, which is released into the circulation [49]. Physiologically, non-pregnant bitches in dioestrus are in a state of pseudopregnancy and there is little hormonal distinction between pregnant and pseudopregnant animals.
The high concentration of circulating progesterone and growth hormone during dioestrus antagonises insulin function and can result in impaired glucose tolerance. This is typically subclinical in younger dogs, whereas overt dioestrous diabetes is more commonly seen in middle-aged and older animals [50]. It is likely that repeated cycles of insulin resistance and glucose intolerance during dioestrus may eventually result in permanently impaired glucose homeostasis. It is possible that clinical signs might resolve if animals were treated with insulin and neutered at the first indication of diabetes, provided that there was some residual beta cell function. Unfortunately, many dogs diagnosed with dioestrus diabetes continue to depend on insulin following ovariohysterectomy, suggesting that there has been considerable loss of beta cells before clinical signs became apparent.
Glycaemic stability is difficult to achieve with insulin therapy in entire female dogs with diabetes due to the varying degrees of insulin resistance during the oestrous cycle. It is therefore recommended that all female dogs undergo ovariohysterectomy as soon as possible after diagnosis of diabetes.
Other types of IRD in dogs
Impaired glucose homeostasis is a feature of other endocrinopathies, including hyperadrenocorticism (Cushing’s disease) and acromegaly, which can lead to overt diabetes in some cases. Hyperglycaemia is typically transient and is reversed when the primary disease has been adequately controlled [27]. We have seen elevated fasting serum C-peptide levels in dogs with hyperadrenocorticism which normalise following treatment with trilostane, a 3β-hydroxysteroid dehydrogenase inhibitor (unpublished data). In a cohort of 221 diabetic dogs from the University of Pennsylvania, USA, 50 (22%) were reported to have evidence of concurrent adrenocortical dysfunction (abnormal low-dose dexamethasone suppression test and/or adrenocorticotrophin stimulation test) [28]. In another study of 60 dogs with confirmed hyperadrenocorticism, 23 were hyperglycaemic with moderate to severe hyperinsulinaemia and five had overt diabetes with relative insulin deficiency [51]. This study suggests that dogs with hyperadrenocorticism can progress from IRD to IDD, if the primary disease is not recognised and treated promptly. In our experience, relatively few dogs presenting with diabetes are subsequently shown to be suffering from concurrent hyperadrenocorticism, although it is possible that this is underdiagnosed.
There is no evidence for a canine equivalent of human type 2 diabetes. Obesity can cause hyperinsulinaemia and glucose intolerance [5] and the effects of insulin resistance appear to be particularly pronounced in dogs fed a diet which is high in saturated fat [52]. Some obese dogs with a fasting hyperinsulinaemia are still able to increase insulin secretion upon intravenous glucose administration, whereas others fail to do so [52]. Although obesity is common in the UK dog population and clearly influences the animal’s ability to utilise glucose, progression to overt diabetes has yet to be documented.
Conclusions
There are several potential causes of beta cell dysfunction in canine diabetes. Type 2 diabetes does not seem to occur in dogs and while most dogs with diabetes are dependent on insulin therapy and are susceptible to diabetic ketoacidosis, the pathogenesis of beta cell loss remains to be defined. Pancreatitis and/or immune-mediated beta cell destruction are likely to be the major underlying causes of IDD in most instances. Autoantibodies to insulin, GAD65 and IA-2 are present in a proportion of dogs but it remains unclear whether these are the cause or consequence of beta cell destruction. Immune-mediated diabetes in dogs might be more analogous to latent autoimmune diabetes of the adult than classic human type 1 diabetes. Further work is needed before we can understand the pathogenesis of canine diabetes and the genetic and environmental factors that contribute to disease susceptibility.
Abbreviations
- DLA:
-
dog leucocyte antigen
- IA-2:
-
islet antigen-2
- IA-2/Cterm:
-
C-terminal region of canine IA-2
- IDD:
-
insulin deficiency diabetes
- IRD:
-
insulin resistance diabetes
References
Von Mering J, Minkowski O (1890) Diabetes mellitus nach pancreas extirpation. Arch Exp Pathol Pharmakol 26:371–381
Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA (1922) Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J 12:141–146
Kim SC, We YM, Lee JH, Kang HY, Han DJ (1988) Impact of purification of pancreatic islets in canine intraportal islet transplantation. Transplant Proc 30:3423–3424
Wilkinson JS (1960) Spontaneous diabetes mellitus. Vet Rec 72:548–553
Mattheeuws D, Rottiers R, Kaneko JJ, Vermeulen A (1984) Diabetes mellitus in dogs: relationship of obesity to glucose tolerance and insulin response. Am J Vet Res 45:98–103
Guptill L, Glickman L, Glickman N (2003) Time trends and risk factors for diabetes mellitus in dogs: analysis of veterinary medical database records (1970–1999). Vet J 165:240–247
Marmor M, Willeberg P, Glickman LT, Priester WA, Cypess RH, Hurvitz AI (1982) Epizootiologic patterns of diabetes mellitus in dogs. Am J Vet Res 43:465–470
Davison LJ, Herrtage ME, Catchpole B (2004) Canine diabetes mellitus in the UK: a study of 253 dogs with naturally occurring disease. Vet Rec 156:467–471
Kennedy LJ, Davison LJ, Barnes A, Isherwood D, Ollier WER, Catchpole B (2003) Susceptibility to canine diabetes mellitus is associated with MHC class II polymorphisms. British Small Animal Veterinary Association Congress 2003, Sci Proc 593 (Abstract)
Foster SJ (1975) Diabetes mellitus—a study of the disease in the dog and cat in Kent. J Small Anim Pract 16:295–315
Atkins CE, Macdonald MJ (1987) Canine diabetes mellitus has a seasonal incidence: implications relevant to human diabetes. Diabetes Res 5:83–87
Hyoty H, Taylor KW (2002) The role of viruses in human diabetes. Diabetologia 45:1353–1361
Hess RS, Ward CR (2000) Effect of insulin dosage on glycaemic response in dogs with diabetes mellitus: 221 cases (1993–1998). J Am Vet Med Assoc 216:217–221
Davison LJ, Podd SL, Ristic JM, Herrtage ME, Parnham A, Catchpole B (2002) Evaluation of two point-of-care analysers for measurement of fructosamine or haemoglobin A1c in dogs. J Small Anim Pract 43:526–532
Hess RS, Kass PH, Van Winkle TJ (2003) Association between diabetes mellitus, hypothyroidism or hyperadrenocorticism and atherosclerosis in dogs. J Vet Intern Med 17:489–494
Gepts W (1965) Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14:619–633
Gepts W, Toussaint D (1967) Spontaneous diabetes in dogs and cats. A pathological study. Diabetologia 3:249–265
Alejandro R, Feldman EC, Shienvold FL, Mintz DH (1988) Advances in canine diabetes mellitus research: etiopathology and results of islet transplantation. J Am Vet Med Assoc 193:1050–1055
Atkins CE, Hill JR, Johnson RK (1979) Diabetes mellitus in the juvenile dog: a report of four cases. J Am Vet Med Assoc 175:362–368
Atkins CE, LeCompte PM, Chin HP, Hill JR, Ownby CL, Brownfield MS (1988) Morphological and immunocytochemical study of young dogs with diabetes mellitus associated with pancreatic islet hypoplasia. Am J Vet Res 49:1577–1581
Imamura T, Koffler M, Helderman JH et al (1988) Severe diabetes induced in subtotally depancreatized dogs by sustained hyperglycaemia. Diabetes 37:600–609
Kramer JW (1981) Animal model of human disease: inherited early-onset, insulin-requiring diabetes mellitus in Keeshond dogs. Am J Pathol 105:194–196
Kramer JW, Klaasen JK, Baskin DG et al (1988) Inheritance of diabetes mellitus in Keeshond dogs. Am J Vet Res 49:428–431
Williams DA (2000) Exocrine pancreatic disease. In: Ettinger SJ, Feldman EC (eds) Textbook of veterinary internal medicine. 5th edn. WB Saunders, Philadelphia, pp 1345–1367
Watson PJ (2003) Exocrine pancreatic insufficiency as an end stage of pancreatitis in four dogs. J Small Anim Pract 44:306–312
Rabinovitch A (1998) An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab Rev 14:129–159
Hoenig M (2002) Comparative aspects of diabetes mellitus in dogs and cats. Mol Cell Endocrinol 197:221–229
Hess RS, Saunders HM, Van Winkle TJ, Ward CR (2000) Concurrent disorders in dogs with diabetes mellitus: 221 cases (1993–1998). J Am Vet Med Assoc 217:1166–1173
Westermarck E, Willberg M (2003) Exocrine pancreatic insufficiency in dogs. Vet Clin North Am Small Anim Pract 33:1165–1179
Hall EJ, Bond PM, McLean C, Batt RM, McLean L (1991) A survey of the diagnosis and treatment of canine exocrine pancreatic insufficiency. J Small Anim Pract 32:613–619
Whitney MS, Boon GD, Rebar AH, Story JA, Bottoms GD (1993) Ultracentrifugal and electrophoretic characteristics of the plasma lipoproteins of Miniature Schnauzer dogs with idiopathic hyperlipoproteinemia. J Vet Intern Med 7:253–260
Mansfield CS, Jones BR, Spillman T (2003) Assessing the severity of canine pancreatitis. Res Vet Sci 74:137–144
Steiner JM (2003) Diagnosis of pancreatitis. Vet Clin North Am Small Anim Pract 33:1181–1195
Simpson JW, Doxey DL (1990) Serum amylase and isoamylase values in dogs with pancreatic disease. Vet Res Commun 14:453–459
Williams DA, Batt RM (1988) Sensitivity and specificity of radioimmunoassay of serum trypsin-like immunoreactivity for the diagnosis of canine exocrine pancreatic insufficiency. J Am Vet Med Assoc 192:195–201
Steiner JM, Williams DA (2003) Development and validation of a radioimmunoassay for the measurement of canine pancreatic lipase immunoreactivity in serum of dogs. Am J Vet Res 64:1237–1241
Davison LJ, Herrtage ME, Steiner JM, Williams DA, Catchpole B (2003) Evidence of anti-insulin autoreactivity and pancreatic inflammation in newly-diagnosed diabetic dogs. J Vet Intern Med 17:395 (Abstract)
Cook AK, Breitschwerdt EB, Levine JF, Bunch SE, Linn LO (1993) Risk factors associated with acute pancreatitis in dogs 101 cases (1985–1990). J Am Vet Med Assoc 203:673–679
Sai P, Debray-Sachs M, Jondet A, Gepts W, Assan R (1984) Anti-beta cell immunity in insulinopenic diabetic dogs. Diabetes 33:135–140
Montgomery TM, Nelson RW, Feldman EC, Robertson K, Polonsky KS (1996) Basal and glucagon-stimulated plasma C-peptide concentrations in healthy dogs, dogs with diabetes mellitus and dogs with hyperadrenocorticism. J Vet Intern Med 10:116–122
Rand JS, Fleeman LM, Farrow HA, Appleton DJ, Lederer R (2004) Canine and feline diabetes mellitus: nature or nurture? J Nutr 134:2072–2080
Zimmet PZ, Tuomi T, Mackay IR et al (1994) Latent autoimmune diabetes in adults (LADA): the role of antibodies to glutamic acid decarboxylase in diagnosis and prediction of insulin dependency. Diabet Med 11:299–303
Hoenig M, Dawe DL (1992) A qualitative assay for beta cell antibodies. Preliminary results in dogs with diabetes mellitus. Vet Immunol Immunopathol 32:195–203
Haines DM, Penhale WJ (1985) Autoantibodies to pancreatic islet cells in canine diabetes mellitus. Vet Immunol Immunopathol 8:149–156
Davison LJ, Weenink SM, Christie MR, Herrtage ME, Catchpole B (2003) Autoantibodies to pancreatic proteins (GAD65 and IA-2) in canine diabetes mellitus. J Vet Intern Med 17:738 (Abstract)
Todd JA, Bell JI, McDevitt HO (1990) HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 329:599–604
Redondo MJ, Eisenbarth GS (2002) Genetic control of autoimmunity in Type 1 diabetes and associated disorders. Diabetologia 45:605–622
Khalil I, d’Auriol L, Gobet M et al (1990) A combination of HLA-DQ beta Asp57-negative and HLA-DQ alpha Arg52 confers susceptibility to insulin-dependent diabetes mellitus. J Clin Invest 85:1315–1319
Selman PJ, Mol JA, Ruttemen GR, van Garderen E, Rijnberk A (1984) Progestin-induced growth hormone excess in the dog originates in the mammary gland. Endocrinology 134:287–292
Eigenmann JE, Eigenmann RY, Rijnberk A et al (1983) Progesterone-controlled growth hormone overproduction and naturally occurring canine diabetes and acromegaly. Acta Endocrinol (Copenh) 104:167–176
Peterson ME, Altszuler N, Nichols CE (1984) Decreased insulin sensitivity and glucose tolerance in spontaneous canine hyperadrenocorticism. Res Vet Sci 36:177–182
Truett AA, Borne AT, Monteiro MP, West DB (1998) Composition of dietary fat affects blood pressure and insulin responses to dietary obesity in the dog. Obes Res 6:137–146
Acknowledgements
We are grateful to all of the owners of diabetic dogs who took part in the study and to their veterinarians for taking the blood samples. Thanks to M. Christie and S. Weenink at King’s College London for their help in setting up the autoantibody assays. Thanks also to L. Kennedy and B. Ollier for performing the DLA genotyping. L. Davison’s PhD was co-supervised by M. Herrtage, Department of Clinical Veterinary Medicine, University of Cambridge and was part funded by Intervet Pharma R&D. We are also grateful to BSAVA Petsavers and the Kennel Club Charitable Trust for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Catchpole, B., Ristic, J.M., Fleeman, L.M. et al. Canine diabetes mellitus: can old dogs teach us new tricks?. Diabetologia 48, 1948–1956 (2005). https://doi.org/10.1007/s00125-005-1921-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1921-1