Abstract
Aims
Glucagon-like peptide-1 (GLP-1) is an insulinotropic hormone released from the gut in response to nutrients. Besides its well-established direct effect on pancreatic beta cells, GLP-1 may also act by activating sensors in the hepatoportal area. We therefore studied the impact of putative GLP-1 sensors in the splanchnic circulation.
Methods
We infused GLP-1 into the portal vein of conscious dogs, while also infusing glucose intraportally or systemically. In the first experiment, we infused glucose intraportally, simulating portal glucose values obtained during a previous mixed-meal test, with or without co-infusion of intraportal GLP-1. In the second experiment, by infusing glucose systemically, with or without intraportal GLP-1, we investigated whether the effects of systemic glucose with or without portal GLP-1 infusion are similar to those observed in the first experiment.
Results
Intraportal infusion of GLP-1 and glucose significantly raised peripheral GLP-1 levels, but did not produce an insulin response different from intraportal glucose alone. However, the resulting peripheral glycaemia was significantly lower compared to glucose infusion alone, and there were elevations in glucagon, cortisol and lactate. In contrast to the portal glucose infusions, there were no significant differences in glucose, insulin, glucagon, cortisol or lactate levels between systemic glucose infusion with or without GLP-1.
Conclusions/Interpretation
Portal GLP-1 and portal glucose, but not systemic glucose, can produce decreased peripheral glucose levels independently of hyperinsulinaemia. This suggests that portal GLP-1 and glucose receptors mediate insulin-independent changes in peripheral glycaemia and determine a strong counter-regulatory response, as reflected by changes in glucagon and cortisol.
Similar content being viewed by others
Introduction
Glucagon-like peptide-1 (GLP-1) is known to increase glucose utilisation via the peptide’s action as an incretin [1–3]. GLP-1 is released from the gut in response to nutrients, and is stored in the intestinal L-cells in the active forms GLP-1(7–37) and GLP-1(7–36) amide [4, 5]. Catalysed by the ubiquitous enzyme dipeptidyl peptidase IV (DPP IV), active GLP-1 degrades rapidly in blood, forming the inactive form GLP-1(9–36 amide), and accounting for the very short in vivo half-life of 1.5–2 min for the active peptide [6, 7]. It is generally held that GLP-1 exerts its effects on glucose turnover by binding to the GLP-1 receptor in pancreatic beta cells and stimulating insulin release [8, 9]. Paradoxically, the hormone’s very short half-life in vivo guarantees that only a small fraction of the secreted active form is able to reach the beta cells intact [6, 10]. Thus, it is possible that alternative mechanism(s) mediate(s) the actions of GLP-1 to enhance glucose utilisation. It has been considered that a target for GLP-1 might be closer to the site of GLP-1 secretion than the pancreatic beta cell. Several investigators have suggested that sensors within the hepatoportal region may be one site of GLP-1 action [11–15]. This latter suggestion appears reasonable, as the hepatoportal region has been identified as a locus for one sensing mechanism for glucose [16–18]. Moreover, it has been shown that infusion of GLP-1 in the portal vein increases the impulse discharge rate in the vagus nerve [19].
The role of GLP-1 sensing in the portal vein remains controversial. Recent work suggesting that GLP-1 can modulate the activity of the hepatoportal sensor in mice [20] has not been confirmed in other studies [21]. Thus, we believed it important to re-examine the effects of portal GLP-1 appearance at levels and with dynamics similar to those seen in vivo. This required examination of potential interactions between the hormone GLP-1 and the nutrient glucose, both of which appear in the portal and systemic circulations during meals.
Materials and methods
Animals
Experiments were performed on six male mongrel dogs (1 year old, BW 27.2±0.7 kg; source: Harlan, Indianapolis, IN, USA) in the conscious relaxed state. All animals were fasted for 12–16 h before the experiments. Dogs were housed under controlled kennel conditions (12 h light, 12 h dark) in the University of Southern California Medical School Vivarium and were fed once a day with standard diet (26% protein, 15% fat and 40% carbohydrate; Prolab canine diet, PMI Nutrition International, Brentwood, MO, USA). Dogs were used for experiments only if judged to be in good health as determined by body temperature, haematocrit, regularity of food intake and direct observation. The Principles of Laboratory Animal Care (NIH publication No. 86-23, revised 1985) were followed and all surgical and experimental procedures were approved by the University of Southern California Institutional Animal Care and Use Committee.
Surgical procedures
At least 1 week prior to the first experiment, chronic catheters (Tygon, ID=0.050″ Norton Plastics, Akron, OH, USA) were implanted under anaesthesia induced with sodium pentothal and maintained with isofluorane. Catheters were placed in the jugular vein (with the tip advanced into the right atrium) for sampling of mixed venous blood (referred to as ‘systemic’) and in the portal vein (4 cm upstream from the porta hepatis) for infusions. All catheters were led subcutaneously to the back of the neck and exteriorised. Catheters were filled with heparinised saline (10 U/ml), were coiled and capped, and then placed in a small bag protected with a stiff collar.
Experimental design
Four different experiments were performed in each animal, in random order (Fig. 1). In two protocols (Protocol 1a and 1b; Fig. 1a) glucose was infused intraportally. Additionally, in Protocol 1b GLP-1 was also infused into the portal vein. The portal glucose infusion rate was variable, designed to simulate the pattern of portal glucose previously observed during a mixed meal in a separate group of dogs (Fig. 2). In a second set of experiments (Protocol 2a and 2b; Fig. 1b), glucose was given systemically, to match the systemic pattern seen during the mixed meal, with (Protocol 2a) or without (Protocol 2b) intraportal GLP-1 infusion.
Experimental design. Protocol 1 (a). Glucose (Protocol 1a), or glucose and GLP-1 (Protocol 1b) were infused intraportally. GINF, glucose infusion rate (intraportal). Protocol 2 (b). Glucose was either infused systemically (Protocol 2a), or it was infused systemically with intraportal GLP-1 (Protocol 2b). The GINF is for systemic glucose infusion. In protocols 1b and 2b, GLP-1 was infused intraportally at a constant rate of 3 pmol·kg−1·min−1
Timing
For all experiments, animals were brought at approximately 07.00 hours to the laboratory and placed in a Pavlov sling. Basal samples were taken from the jugular vein at −20 and −10 min. Infusions (glucose±GLP-1 ) started at 0 min and continued for 240 min. Blood was sampled from the jugular vein catheter at 5, 10, 20, 30, 40, 60, 90, 120, 150, 180, 210 and 240 min.
Protocol 1a and 1b: portal glucose infusion with or without intraportal GLP-1 infusion
In protocol 1a, beginning at 0 min, glucose (20% hydrated dextrose, anhydrous concentration 181.8 mg/ml; B Braun, Irvine, CA, USA) was infused directly into the portal vein at time-varying rates (Fig. 1a) to simulate the portal glucose concentration pattern previously observed during the mixed meal (Fig. 2).
In protocol 1b, variable-rate glucose was infused intraportally at rates identical to Protocol 1a. In addition, starting at 0 min, constant-rate GLP-1 (human GLP-1 [7–36] amide; Bachem Bioscience, King of Prussia, PA, USA; dissolved in saline with 1% dog albumin) was also infused intraportally (3 pmol·kg−1·min−1).
Protocol 2a and 2b: systemic glucose infusion with or without intraportal GLP-1 infusion
In all experiments requiring systemic infusion of glucose, an intracatheter (BD Intracath 19 GA; Becton-Dickinson, Sandy, UT, USA) was placed in the saphenous vein and secured. Basal sampling was initiated after the dog had rested for 20 min.
Beginning at 0 min (Protocol 2a), glucose was infused in the saphenous vein at time-varying rates (Fig. 1b) to simulate the systemic glucose concentration pattern previously observed during the mixed meal (Fig. 2).
In Protocol 2b, glucose was infused systemically at rates identical to Protocol 2a. In addition, starting at 0 min, constant-rate GLP-1 (human GLP-1 [7–36] amide) was infused intraportally (3 pmol kg−1 min−1).
Blood sampling
Samples for the determination of glucose, insulin, lactate, NEFA, growth hormone and cortisol were collected into chilled tubes coated with lithium fluoride and heparin, and containing 50 μl EDTA 2% (w/vol) (Sigma, St. Louis, MO, USA). Samples for C-peptide and glucagon assays were collected into tubes containing 25 μl EDTA, 50 μl Trasylol (10,000 KIU/ml; Serological Proteins, Kankakee, IL, USA) and 50 μl 0.1 mmol/l diprotin A (Sigma). Samples for the assay of active GLP-1 were collected into tubes containing EDTA and 10 μl/ml blood DPP-IV inhibitor (Linco Research., St Charles, MO, USA). All samples were stored on ice until centrifugation, after which plasma was separated and stored at −80°C until analysis. To prevent triglyceride breakdown, the NEFA samples were kept on ice and either immediately assayed or kept at −80°C for a limited time until NEFA determination.
Assays
Blood glucose and lactate concentrations were determined during experiments using a YSI 2300 autoanalyser (Yellow Springs Instruments, Yellow Springs, OH, USA). The remaining plasma was stored at −80°C for further analysis. Insulin was measured using a human insulin enzyme-linked immunoassay kit (Linco Research) adapted in our laboratory for dog plasma. The assay uses two monoclonal antibodies that bind to different epitopes on the insulin molecule and do not bind to proinsulin. The ELISA assay cross-reacts with canine insulin and has been previously validated in our laboratory [22]. C-peptide, glucagon, cortisol and growth hormone were measured using radioimmunoassay kits (Linco Research). Active GLP-1 was measured by ELISA (Linco Research). Plasma NEFA concentrations were determined using an enzymatic colorimetric assay based on the acylation of CoA (NEFA C kit; Wako Chemicals, Neuss, Germany).
Calculations and statistical analysis
Insulin secretion was calculated from the C-peptide data using the deconvolution analysis based on a two-compartment model of C-peptide kinetics, as previously described [23].
Results are presented as means±SE. Basal (fasting) values were calculated as the mean of the two samples taken at −20 and −10 min. Changes relative to basal were calculated using repeated measures ANOVA with Dunnett’s post-test. Repeated measures ANOVA with Bonferroni post-test was used to compare time lines for infusion experiments. Paired t-tests were used to identify the significantly different time-point pairs. AUCs above basal were calculated using the trapezoid rule. All differences were considered statistically significant at a p value of less than <0.05.
Results
Protocol 1: portal glucose infusion with or without portal GLP-1 infusion
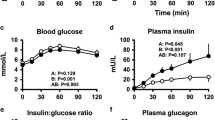
Infusion of glucose in the portal vein produced a small, but significant increment in systemic glucose, from a basal level of 5.29±0.23 mmol/l to a maximum of 5.98±0.29 mmol/l (p<0.01; Fig. 3a). The increase in glucose concentrations was restrained by the significant increase in systemic insulin from 37±8 pmol/l to 98±21 pmol/l (p<0.01; Fig. 3b). As expected with intravenous glucose, active GLP-1 in systemic blood was not changed (9±3 pmol/l). The increase in insulin secretion was confirmed by a rise in C-peptide, from 0.16±0.04 nmol/l to 0.26±0.07 nmol/l (Fig. 3c). The calculated rate of intraportal insulin secretion, based on C-peptide deconvolution analysis, also increased (Fig. 3d). NEFA were suppressed, while cortisol, glucagon, lactate and growth hormone remained unchanged (Table 1, Fig. 4). Thus, the infusion of glucose intraportally yielded a significant insulin secretory response and a small increment in plasma glucose with little change in other parameters.
The addition of intraportal GLP-1 to intraportal glucose infusion raised systemic GLP-1 levels by 11 pmol/l, from 5±0 to 16±4 pmol/l, and resulted in a very different systemic glucose profile than that produced by portal glucose alone (Fig. 3a). The concentration of systemic glucose during GLP-1 infusion was significantly lower than without GLP-1. In fact, in the second half of the experiment, the glucose levels were significantly below basal (AUC120–240 min=−51.50±19.02 mmol·l−1·min−1 vs AUC0–120 min=20.05±7.75 mmol·l−1·min−1; p<0.05). It is conceivable that insulin secretion could have accounted for the lower glycaemia seen with portal infusion of GLP-1. However, there were no measurable differences in insulin or C-peptide between Protocols 1a and 1b (p=0.47 and p=0.10 respectively; Fig. 3b, c). The insulin AUC above basal for portal glucose and portal GLP-1 infusion (5,017±889 pmol·l−1·min−1) was not higher than that above basal for infusion portal glucose alone (5,576±1,194 pmol·l−1·min−1; p=0.76). Similarly, the C-peptide AUC above basal for portal glucose plus GLP-1 (7±2 nmol·l−1·min−1) was lower than AUC above basal for infusion of portal glucose alone (12±4 nmol·l−1·min−1; p=0.33). When we calculated intraportal insulin appearance, based on C-peptide deconvolution, we found no significant difference between insulin intraportal appearance during portal glucose infusion alone and infusion of portal glucose plus GLP-1 (p=0.2, Fig. 3d). Failing an enhancement in insulin release, it appears that the combination of intraportal GLP-1 and glucose was able to reduce peripheral glycaemia, independently of hyperinsulinaemia.
The levels of the counter-regulatory hormones glucagon and cortisol increased dramatically in Protocol 1b, whereas they failed to change in Protocol 1a (Fig. 4). Both glucagon and cortisol quadrupled in the presence of portal GLP-1, with glucagon increasing from 52±6 ng/l to a maximum of 191±48 ng/l at 240 min (p<0.01), while cortisol increased from 87±17 nmol/l to a maximum of 360±53 nmol/l at 180 min (p<0.01; Fig. 4). Glucagon and cortisol appeared to increase early at around 10 min; however, this increase did not achieve statistical significance. Growth hormone did not increase significantly during co-infusion of GLP-1, and there was no difference between the levels of growth hormone with or without GLP-1 (Table 1).
NEFA suppression was similar in Protocol 1a and 1b (Table 1). The mean lactate concentration increased during co-infusion of GLP-1, from 1.2±0.2 mmol/l to 1.7±0.3 mmol/l, but this increase did not reach significance (p=0.08; Fig. 4c).
The plasma concentrations of glucagon, cortisol and lactate in Protocol 1b were temporally and significantly correlated with the level of systemic glycaemia. The relationship is illustrated in Fig. 5, which shows the correlation between glucose and glucagon levels, glucose and cortisol levels, or glucose and lactate levels, at each particular time point for the duration of the experiment (r=−0.79, p<0.01 for glucagon; r=−0.71, p<0.01 for cortisol; r=−0.73, p<0.01 for lactate).
Protocol 2: systemic glucose infusion with or without portal GLP-1 infusion
To rule out the possibility that the lower systemic glycaemia and increases in counter-regulatory hormones seen with Protocol 1 were due to a direct peripheral effect of glucose and GLP-1, we infused glucose systemically (in Protocol 2a and 2b) to match the modest systemic hyperglycaemia observed in Protocol 1a, without or with portal infusion of GLP-1.
When glucose was infused systemically (without GLP-1 infusion), we measured an increase of 0.4 mmol/l in systemic glucose concentration, from 5.41±0.14 mmol/l to 5.81±0.18 mmol/l (Fig. 6a). The small increase in glycaemia with systemic infusion (Protocol 2a) was not significantly different from the increase in systemic glucose produced with intraportal glucose infusion (Protocol 1a) (p=0.56). There was a small insulin response (39±10 pmol/l to 86±16 pmol/l at 30 min; Fig. 6b), an increase similar to that observed with Protocol 1a (p=0.32). In the two protocols we also measured very similar increases in C-peptide, from 0.21±0.03 nmol/l to 0.25±0.04 nmol/l (Fig. 6c). GLP-1 levels remained constant at 12±4 pmol/l. The small increases in mean glucagon, cortisol, growth hormone and lactate were not statistically different from basal (Fig. 6d–f, Table 1).
The addition of intraportal GLP-1 increased systemic GLP-1 levels by 9 pmol/l, but had no significant effect on any of the other parameters measured. Glucose, insulin, C-peptide, NEFA, lactate, cortisol, glucagon and growth hormone levels were not significantly different from those measured with systemic glucose infusion alone (Fig. 6a–f; Table 1). On the contrary, insulin levels seemed slightly lower with GLP-1 than without it. The AUC above basal for insulin was 4,264±1,892 pmol·l−1·min−1 in the presence of GLP-1 and 4,577±1,355 pmol·l−1·min−1 without GLP-1 (p=0.91). The C-peptide concentration exhibited a similar pattern: the AUC above basal was 10±2 nmol·l−1·min−1 for the glucose infusion and 7±3 nmol·l−1·min−1 for the infusion of glucose plus GLP-1 (p=0.54). There were no differences in calculated intraportal insulin appearance between glucose infusions with or without GLP-1 (data not shown). Thus, the lower glycaemia and increases in counter-regulatory hormones observed in Protocol 1b cannot be explained by a direct synergistic interaction between glucose and GLP-1 at the pancreatic beta cells.
Discussion
The aim of our study was to assess the impact of portal glucose and portal GLP-1 on glucose regulation. To this end, we infused glucose intraportally with or without co-infusion of intraportal GLP-1. The addition of GLP-1 produced lower systemic glucose levels than those obtained with intraportal glucose infusion alone, suggesting that the combination of GLP-1 and glucose in the portal circulation increased the disappearance rate of systemic glucose and/or suppressed hepatic glucose output. Interestingly, systemic insulin levels during the combined intraportal glucose plus GLP-1 infusion were not different from those produced by glucose alone. Although we did not measure portal insulin, the similarity of insulin levels in the two protocols was confirmed by comparable C-peptide levels, as well as similar rates of insulin secretion as calculated from C-peptide deconvolution analysis, suggesting that the lower systemic glucose induced by the addition of portal GLP-1 was independent of any corresponding increase in insulin secretion. Although GLP-1 can be expected to increase insulin secretion in the presence of glucose, there is considerable controversy about whether physiological levels of GLP-1 are able to potentiate insulin secretion in a state of euglycaemia [24, 25]. It is therefore possible that the low increase in systemic glucose during the portal infusion precluded a systemic insulinotropic effect of GLP-1. This interpretation is supported by the result of the systemic glucose and GLP-1 infusion, when in a similar glycaemic environment, the addition of GLP-1 did not result in enhanced insulin secretion. It appears from the time lines of insulin and C-peptide concentrations during portal infusions that the addition of GLP-1 may have contributed to an earlier insulin response to glucose. However, it is hard to determine the significance of this result. Systemic glucose drops rapidly (<20 min) in response to a rise in insulin [18], therefore we believe it is unlikely that the early insulin increase is responsible for the lower glucose levels in the last 120 min of the experiment. Moreover, if early insulin did contribute, the insulin pattern should also have produced lower levels of glycaemia in the systemic glucose infusion.
Several studies have indicated that GLP-1 might have an insulin-independent effect on glucose homeostasis [26–32], while other researchers suggested that GLP-1 did not acutely affect glucose metabolism independently of insulin [33–37]. These conflicting results may be a consequence of the large variability in the model and methods used: healthy vs diabetic, diabetic hyperglycaemic vs diabetic maintained euglycaemic, clamp vs IVGTT etc. An important factor that might explain the lack of an insulin-independent effect of GLP-1 in many studies is the delivery route. The majority of studies use peripheral infusions of glucose and GLP-1, therefore supplying GLP-1 in a non-physiological way and in this manner possibly under-stimulating any potential receptor in the splanchnic circulation, including the portal vein. Our study shows that intraportal GLP-1 infusion in the presence of portal hyperglycaemia has an insulin-independent effect; moreover, our results demonstrate that the effect is present at physiological glucose and GLP-1 concentrations, and while the insulin-secreting ability of the pancreas is intact, making it more plausible that this type of regulatory mechanism plays a part in glucose regulation under normal conditions.
The physiological impact of the presence of portal GLP-1 cannot be dissociated from the role of portal glucose, since during a mixed meal both these factors are present in the portal area. While in mice the infusion of portal glucose at a rate equivalent to postabsorptive endogenous glucose production (EGP) induced hypoglycaemia [14, 20], the result was not replicated in humans [38] or dogs [39]. In our study the infusion of intraportal glucose at rates similar to fasting EGP did not have an overtly hypoglycaemic effect. However, we did obtain lower glucose, approaching hypoglycaemia, and a strong counter-regulatory response, when both glucose and GLP-1 were infused. This is consistent with other results indicating a functional and possibly structural relationship between portal glucose and GLP-1 sensors [14], and suggests that the activation of glucose and GLP-1 sensors in the portal area might be a fundamental requirement for glucose regulation in response to a meal.
During the combined intraportal glucose and GLP-1 infusion we measured dramatic increases of glucagon and cortisol in the systemic circulation. This may seem paradoxical, since it is generally accepted that GLP-1 acting on the pancreas increases insulin secretion but reduces or leaves glucagon unchanged [40]. It has been shown that the previous assertion is true under conditions of hyper- and euglycaemia. However, during hypoglycaemia counter-regulatory mechanisms will override the glucagon suppression [41]. In our study, the hypoglycaemic threshold of about 4 mmol/l that has been established for counter-regulation [42] was only reached in one animal, while the average values, though below basal, remained above the threshold. However, it has been reported that a non-hyperinsulinaemic-induced decrease in glucose of 0.6 mmol/l to 5.2 mmol/l can result in a fall of plasma insulin and an increase in glucagon, indicating that in the dog the pancreatic alpha cell is very sensitive to a drop in glucose [43]. Moreover, the increase in lactate levels during GLP-1 infusion might signal an increase in gluconeogenic substrates that are mobilised to prevent hypoglycaemia. Though growth hormone levels did not increase significantly during GLP-1 infusion, the concerted response of increases in glucagon and cortisol, a reduction in insulin levels to below basal (starting around 120 min), and increases in lactate levels indicates a true counter-regulatory response that is determined by the presence of GLP-1 and glucose in the portal vein.
In conclusion, this study describes the novel finding that portal GLP-1 and portal glucose, but not systemic glucose, can produce lower glycaemia independently of insulin secretion, this lower glycaemia being accompanied by increases in glucagon and cortisol. The data suggest that the effect is mediated by glucose/GLP-1 receptors in the portal vein. It appears that GLP-1 contributes to glucose regulation not only through its well-known insulinotropic effect, but also via an insulin-independent effect. This dual action makes GLP-1 a very good candidate for therapy in diabetes, where both the insulin secretory effect and hypoglycaemic effect could be efficacious in regulating blood glucose.
Abbreviations
- DPP IV:
-
Dipeptidyl peptidase IV
- EGP:
-
Endogenous glucose production
- GLP-1:
-
Glucagon-like peptide-1
References
Holst JJ (2002) Therapy of type 2 diabetes mellitus based on the actions of glucagon-like peptide-1. Diabetes/Metab Res Rev 18:430–441
Nauck M (1999) Is glucagon-like peptide 1 an incretin hormone? Diabetologia 42:373–379
Drucker DJ (2001) Mini review: the glucagon-like peptides. Endocrinology 142:521–527
Suda K, Takahashi H, Fukase N, Manaka H, Tominaga M, Sasaki H (1989) Distribution and molecular forms of glucagon-like peptide in the dog. Life Sci 45:1793–1798
Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ (1994) Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide 1 in humans. Diabetes 43:535–539
Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ (1996) Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol, Endocrinol Metab 271:E458–E464
Kieffer TJ, Mcintosh CHS, Pederson RA (1995) Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide-1 in vitro and in vivo by dipeptidyl peptidase-IV. Endocrinology 136:3585–3596
Drucker DJ (2002) Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology 122:531–544
Holst JJ (1999) Glucagon-like peptide 1 (GLP-1): an intestinal hormone, signalling nutritional abundance, with an unusual therapeutic potential. Trends Endocrinol Metab 10:229–235
Hansen L, Deacon CF, Orskov C, Holst JJ (1999) Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase iv in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140:5356–5363
Nishizawa M, Nakabayashi H, Uchida K, Nakagawa A, Niijima A (1996) The hepatic vagal nerve is receptive to incretin hormone glucagon-like peptide-1, but not to glucose-dependent insulinotropic polypeptide, in the portal vein. J Auton Nerv Syst 61:149–154
Nishizawa M, Nakabayashi H, Kawai K et al. (2000) The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. J Auton Nerv Syst 80:14–21
Balkan B, Li X (2000) Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol, Regul Integr Comp Physiol 279:R1449–R1454
Burcelin R, Da Costa A, Drucker D, Thorens B (2001) Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 50:1720–1728
Nishizawa M, Moore MC, Shiota M et al. (2003) Effect of intraportal glucagon-like peptide-1 on glucose metabolism in conscious dogs. Am J Physiol, Endocrinol Metab 284:E1027–E1036
Hevener AL, Bergman RN, Donovan CM (2000) Portal vein afferents are critical for the sympathoadrenal response to hypoglycemia. Diabetes 49:8–12
Donovan CM, Halter JB, Bergman RN (1991) Importance of hepatic glucoreceptors in sympathoadrenal response to hypoglycemia. Diabetes 40:155–158
Hamilton-Wessler M, Bergman RN, Halter JB, Watanabe RM, Donovan CM (1994) The role of liver glucosensors in the integrated sympathetic response induced by deep hypoglycemia in dogs. Diabetes 43:1052–1060
Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Nijima A (1996) Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol, Endocrinol Metab 34:E808–E813
Burcelin R, Dolci W, Thorens B (2000) Portal glucose infusion in the mouse induces hypoglycemia: evidence that the hepatoportal glucose sensor stimulates glucose utilization. Diabetes 49:1635–1642
Dardevet D, Moore MC, Neal D, DiCostanzo CA, Snead W, Cherrington AD (2004) Insulin-independent effects of GLP-1 on canine liver glucose metabolism: duration of infusion and involvement of hepatoportal region. Am J Physiol, Endocrinol Metab 287:E75–E81
Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN (1996) Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J Clin Invest 97:1497–1503
Watanabe RM, Bergman RN (2000) Accurate measurement of endogenous insulin secretion does not require separate assessment of C-peptide kinetics. Diabetes 49:373–382
Nauck MA, Niedereichholz U, Ettler R et al. (1997) Glucagon-like peptide 1inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol, Endocrinol Metab 273:E981–E988
Ryan AS, Egan JM, Habener JF, Elahi D (1998) Insulinotropic hormone glucagon-like peptide-1-(7–37) appears not to augment insulin-mediated glucose uptake in young men during euglycemia. J Clin Endocrinol Metab 83:2399–2404
Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S (1992) Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 326:1316–1322
Meneilly GS, McIntosh CHS, Pederson RA et al. (2001) Effect of glucagon-like peptide 1 on non-insulin-mediated glucose uptake in the elderly patient with diabetes. Diabetes Care 24:1951–1956
Freyse EJ, Becher T, El Hag O, Knospe S, Goke B, Fischer U (1997) Blood glucose lowering and glucagonostatic effects of glucagon-like peptide 1 in insulin-deprived diabetic dogs. Diabetes 46:824–828
Sandhu H, Wiesenthal SR, MacDonald PE et al. (1999) Glucagon-like peptide 1 increases insulin sensitivity in depancreatized dogs. Diabetes 48:1045–1053
Shalev A, Ninnis R, Keller U (1998) Effects of glucagon-like peptide 1 (7–36 amide) on glucose kinetics during somatostatin-induced suppression of insulin secretion in healthy men. Horm Res 49:221–225
D’Alessio DA, Kahn SE, Leusner CR, Ensinck JW (1994) Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest 93:2263–2266
D’Alessio DA, Prigeon RL, Ensinck JW (1995) Enteral enhancement of glucose disposition by both insulin-dependent and insulin-independent processes. A physiological role of glucagon-like peptide 1. Diabetes 44:1433–1437
Orskov L, Holst JJ, Moller J et al. (1996) GLP-1 does not acutely affect insulin sensitivity in healthy man. Diabetologia 39:1227–1232
Toft-Nielsen M, Madsbad S, Holst JJ (1996) The effect of glucagon-like peptide 1 (GLP-1) on glucose elimination in healthy subjects depends on the pancreatic glucoregulatory hormones. Diabetes 45:552–556
Larsson H, Holst JJ, Ahren B (1997) Glucagon-like peptide-1 reduces hepatic glucose production indirectly through insulin and glucagon in humans. Acta Physiol Scand 160:413–422
Hvidberg A, Nielsen MT, Hilsted J, Orskov C, Holst JJ (1994) Effect of glucagon-like peptide-1 (proglucagon 78–107 amide) on hepatic glucose production in healthy man. Metabolism 43:104–108
Freyse EJ, Knospe S, Becher T, El Hag O, Goke B, Fischer U (1999) Glucagon-like peptide-1 has no insulin-like effects in insulin-dependent diabetic dogs maintained normoglycemic and normoinsulinemic. Metabolism 48:134–137
Zangeneh F, Basu R, Shah P, Arora P, Camilleri M, Rizza RA (2003) Enteral infusion of glucose at rates approximating EGP enhances glucose disposal but does not cause hypoglycemia. Am J Physiol, Endocrinol Metab 285:E280–E286
Moore MC, Cardin S, Edgerton DS et al. (2004) Unlike mice, dogs exhibit effective glucoregulation during low-dose portal and peripheral glucose infusion. Am J Physiol, Endocrinol Metab 286:E226–E233
Holst JJ, Gromada J (2004) Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol, Endocrinol Metab 287:E199–E206
Nauck MA, Heimesaat MM, Behle K et al. (2002) Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 87:1239–1246
Cryer PE (1997) Hierarchy of physiological responses to hypoglycemia: relevance to clinical hypoglycemia in type I (insulin-dependent) diabetes mellitus. Horm Metab Res 29:92–96
Flattem N, Igawa K, Shiota M, Emshwiller MG, Neal DW, Cherrington AD (2001) Alpha- and beta cell responses to small changes in plasma glucose in the conscious dog. Diabetes 50:367–375
Acknowledgements
This work was supported by research grants (to R.N. Bergman) from the National Institutes of Health (DK 27619 and DK 29867), an American Diabetes Association mentor-based fellowship support to V. Ionut and a pre-doctoral training grant from the National Institutes of Aging (T32-AG-00093) to I. F.Liberty. We are especially grateful to Dr Erlinda Kirkman for surgery, to Elza Demirchyan, Sonia Manjikian and Rita Thomas for technical assistance, and to Ed Zuniga for animal care and assistance with the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ionut, V., Hucking, K., Liberty, I.F. et al. Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia 48, 967–975 (2005). https://doi.org/10.1007/s00125-005-1709-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1709-3