Abstract
Aims/hypothesis
We examined the prevalence of islet autoantibodies and their relationship to glycaemic control over 10 years in patients diagnosed clinically with new-onset type 2 diabetes.
Methods
Patient clinical characteristics and autoantibody status were determined at entry to the UK Prospective Diabetes Study (UKPDS) before randomisation to different glucose control policies. Patients were followed for 10 years.
Results
Data available on 4,545 of the 5,102 UKPDS patients showed that 11.6% had antibodies to at least one of three antigens: islet cell cytoplasm, glutamic acid decarboxylase and islet autoantibody 2A (IA-2A). Autoantibody-positive patients were younger, more often Caucasian and leaner, with lower beta cell function and higher insulin sensitivity than autoantibody-negative patients. They also had higher HbA1c, and HDL-cholesterol levels, and lower blood pressure, total cholesterol and plasma triglyceride levels. Despite relative hyperglycaemia, autoantibody-positive patients were less likely to have the metabolic syndrome (as defined by the National Cholesterol Education Program Adult Treatment Program III), reflecting a more beneficial overall risk factor profile. Of 3,867 patients with post-dietary run-in fasting plasma glucose (FPG) values between 6.0 and 14.9 mmol/l and no hyperglycaemic symptoms, 9.4% were autoantibody-positive, compared with 25.1% of 678 patients with FPG values of 15.0 mmol/l or higher, or hyperglycaemic symptoms. In both groups, no differences were seen between those with and without autoantibodies in changes to HbA1c over time, but autoantibody-positive patients required insulin treatment earlier, irrespective of the allocated therapy (p<0.0001).
Conclusions/interpretation
Autoantibody-positive patients can be treated initially with sulphonylurea, but are likely to require insulin earlier than autoantibody-negative patients.
Similar content being viewed by others
Introduction
Type 2 diabetes is a heterogeneous disorder. In northern European populations, for example, 9–12% of adults clinically diagnosed with type 2 diabetes have a phenotype similar to that of classical type 1 diabetes and immunoserological markers of autoimmune beta cell damage [1, 2]. This percentage is higher in younger patients. In patients with newly diagnosed type 2 diabetes, recruited into the United Kingdom Prospective Diabetes Study (UKPDS), 35% of those aged 25–34 years had islet cell cytoplasm antibodies (ICA) and/or autoantibodies to glutamic acid decarboxylase (GADA), compared with 2% in the 55- to 65-year age group [3]. Autoantibody positivity was associated with relatively poor glycaemic control and rapid progression to insulin therapy, with 58% of UKPDS patients who were autoantibody-positive (Ab+ve) requiring insulin on a clinical basis within 6 years of diagnosis, regardless of age [3].
There is continued uncertainty concerning the clinical relevance of islet autoantibody status in clinically diagnosed type 2 diabetes [4]. The UKPDS, which randomised patients with newly diagnosed type 2 diabetes to different glucose control modalities irrespective of islet autoantibody status, provides an opportunity to examine objectively the effect of autoantibody status on glycaemic response to therapy and to assess the association between autoantibody status and cardiovascular risk.
Subjects and methods
Patients
The UKPDS was carried out according to the Helsinki guidelines. All patients gave informed consent to participation in the study and the protocol was approved by the Research Ethics Committee in each of the 23 centres. A total of 5,102 patients aged 25–65 years with type 2 diabetes newly diagnosed by their general practitioner and a fasting plasma glucose (FPG) higher than 6.0 mmol/l on two subsequent occasions were recruited as described previously [5]. Exclusion criteria included: (1) severe vascular disease (myocardial infarction in the past year, current angina or heart failure); (2) accelerated hypertension; (3) proliferative or pre-proliferative retinopathy; (4) renal failure with plasma creatinine higher than 175 mol/l; (5) other life-threatening disease such as cancer; (6) an illness requiring systemic steroids; (7) an occupation precluding insulin treatment; (8) unfamiliarity with English; and (9) ketonuria greater than 3 mmol/l suggestive of type 1 diabetes.
All patients entered a 3- to 4-month dietary run-in period, during which they were advised to take a diet containing 50% carbohydrate, and with low saturated fat content, moderately high fibre content, and a reduced total energy content if obese. Of the 5,102 patients, 4,545 with FPG levels of 6.0 mmol/l or higher after the dietary run-in were included in the present study (Fig. 1). This meant that 149 subjects with FPG levels after the run-in period persistently below 6.0 mmol/l and 408 overweight individuals randomised initially to metformin were excluded.
Following the dietary run-in period, 3,867 of the 4,545 patients had FPG values between 6.0 and 14.9 mmol/l without hyperglycaemic symptoms, of whom 696 (18%) were randomised after an extended dietary run-in period with a median duration of 2.0 [interquartile range (IQR) 1.0–3.5] years. They were randomised to a conventional glucose control policy primarily with diet (n=1,138), or to a more intensive glucose control therapy primarily with sulphonylurea (n=1,573), or to insulin (n=1,156) therapy (Fig. 1). The 678 patients whose FPG values remained at or above 15.0 mmol/l or who had hyperglycaemic symptoms were allocated only to the more intensive glucose control policy and randomised to sulphonylurea (n=393) or insulin (n=285) therapy (Fig. 1). Baseline characteristics for the randomised patient groups did not differ by allocated therapy [6, 7]. Frequency of follow-up and criteria for intensifying or changing therapy under the UKPDS protocol have been published previously [5].
Methods
ICA and GADA were measured as reported previously [3] on samples taken at or soon after diagnosis of diabetes. Only 1.2% of samples were taken more than 2 years after diagnosis. ICA positivity was taken as five JDF units or more, GADA positivity as 20 reference units or more. The sensitivity and specificity of the assays for the diagnosis of type 1 diabetes at these thresholds were 88 and 97% for ICA [8] and 75 and 100% for GADA under the conditions of the JDF Combinatorial Autoantibody Workshop of 1995 [9]. Plasma samples were subsequently screened for islet autoantibody 2A (IA-2A) using a combined radiobinding assay [10, 11]. The thresholds for positivity were determined from the 99th centile of control subjects, and corresponded to one unit for IA-2A.
Fasting plasma glucose was measured in each UKPDS centre, with a monthly quality assurance scheme showing a coefficient of variation of less than 4%. HbA1c (normal range 4.5–6.2%) and other biochemical measurements were assayed as described previously [6, 12]. Homeostasis model assessment (HOMA) was employed to derive estimates of beta cell function (%B) and insulin sensitivity (%S) from simultaneous FPG and fasting plasma insulin measurements at the end of the dietary run-in period [13], using the HOMA2 calculator (http://www.dtu.ox.ac.uk) implementation of the HOMA2 model [14]. Blood pressure was measured with the patient seated, using an automated Copal UA251 or Takeda sphygmomanometer. A Hawksley random zero sphygmomanometer with a large cuff was used if the circumference of the upper arm exceeded 33 cm or if the patient was in atrial fibrillation [6].
Statistical analysis
Autoantibody status was defined as positive if any of ICA, GADA or IA-2A were positive. Otherwise it was defined as negative. Patients were defined as ‘requiring insulin’ in the UKPDS protocol if they had FPG values above 15.0 mmol/l or hyperglycaemic symptoms despite maximally tolerated sulphonylurea and metformin. The presence of the metabolic syndrome was determined using the National Cholesterol Education Program Adult Treatment Program III (NCEP ATPIII) criteria [15]. Statistical analyses were performed using SAS v8.2 (SAS Institute, Cary, NC, USA). Data are reported as means (± standard deviation), geometric mean (one standard deviation interval), median (interquartile range) or percentages. Change over time is reported as means (95% CI). Comparisons between autoantibody status groups used two-sample t-tests, or Wilcoxon signed-rank tests for non-normally distributed data. Categorical comparisons used the chi square test and odds ratios were calculated from the contingency tables. The Breslow–Day test for homogeneity of odds ratios was used as appropriate. Comparisons between conventional and intensive blood glucose control policies and autoantibody status groups used two-way factorial ANOVA with non-normally distributed data transformed to normality. Analyses in relation to therapy were on an intention-to-treat basis. A Kaplan–Meier survival analysis of time to requiring insulin therapy according to autoantibody status was carried out and relative risks (RR) with 95% CIs estimated as hazard ratios from a proportional hazards model. A conventional significance of p<0.05 was used throughout.
Results
Patient characteristics
Overall, 526 (11.6%) of the 4,545 patients studied were Ab+ve at diagnosis: 66 (1.5%) had ICA alone, 272 (6.0%) had GADA alone, 12 (0.3%) had IA-2A alone, 95 (2.1%) had ICA and GADA, three (0.1%) had ICA and IA-2A, ten (0.2%) had GADA and IA-2A, and 68 (1.5%) had all three antibodies.
When compared with Ab−ve patients (Table 1), those who were Ab+ve were younger (p<0.0001) and tended to be female, rather than male. They were also more frequently Caucasian (p<0.0001). After the dietary run-in period Ab+ve patients were leaner (p<0.0001), with lower HOMA %B (p<0.0001) and higher HOMA %S (p<0.0001). They also had higher HbA1c (p<0.0001), and lower systolic (p<0.0001) and diastolic (p<0.0001) blood pressure, plasma cholesterol (p=0.0030) and plasma triglyceride levels (p<0.0001). Ab+ve patients were less likely than Ab−ve patients to have the metabolic syndrome (42 vs 59% respectively, p<0.0001). Adjustment for age, sex and ethnicity did not alter the significance of these relationships.
Of the 3,867 patients with FPG values between 6.0 and 14.9 mmol/l and no hyperglycaemic symptoms following the dietary run-in period, 9.4% were Ab+ve compared with 25.1% of the 678 patients with FPG values of 15.0 mmol/l or higher, or hyperglycaemic symptoms. The odds ratio for Ab+ve patients at diagnosis continuing to have FPG values of 15.0 mmol/l or higher despite dietary intervention was 2.6 (95% CI 2.2–3.0). The proportion of Ab+ve patients with the metabolic syndrome was the same for those achieving FPG values between 6.0 and 14.9 mmol/l and those with FPG values of 15.0 mmol/l or higher (43 vs 41% respectively, p=0.31).
Response to blood glucose-lowering therapies
Mean changes between baseline values following the dietary run-in period and at 10 years are shown in Table 2 for 2,132 patients with data at both times, categorised according to FPG values after the dietary run-in and antibody status. In those with FPG values between 6.0 and 14.9 mmol/l and no hyperglycaemic symptoms, Ab+ve patients gained more weight (p=0.0023), and tended to have a greater rise in plasma HDL-cholesterol. No differences were seen in HbA1c, FPG, systolic blood pressure, LDL-cholesterol or triglycerides. In those with FPG values of 15.0 mmol/l or higher, or with hyperglycaemic symptoms, Ab+ve patients gained more weight (p=0.0017) but no other differences were observed.
Figures 2 and 3 show the body weight and HbA1c over 10 years according to FPG values after the dietary run-in, autoantibody status and randomised therapy. There was a progressive rise in HbA1c in all therapies, with no interaction between autoantibody status and allocated therapy, in those with FPG values after the dietary run-in of 6.0–14.9 mmol/l or 15.0 mmol/l or higher (p=0.42 and p=0.33 respectively).
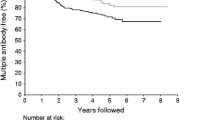
Figure 4 shows a Kaplan–Meier analysis of time to requiring insulin therapy after randomisation to diet or sulphonylurea therapy, according to autoantibody status. Ab+ve patients more rapidly became insulin-requiring than those who were Ab−ve, irrespective of FPG values after the dietary run-in or the allocated therapy for glycaemia. For those with FPG values between 6.0 and 14.9 mmol/l the RRs (95% CI) for Ab+ve compared with Ab−ve were conventional treatment 2.8 (1.8–4.4), sulphonylurea 3.7 (2.8–4.9). The Ab+ve groups receiving conventional and sulphonylurea treatment appeared to be significantly different (log rank p value 0.039), but this analysis is confounded by therapy allocation. The groups had similar baseline characteristics and the difference in time to requiring insulin reflects the protocol-driven therapy changes, as patients allocated to conventional therapy were required to pass through diet and sulphonylurea before being given insulin therapy. For those with FPG values higher than 15.0 mmol/l and allocated to sulphonylurea, the RR (95% CI) for Ab+ve compared with Ab−ve were 4.0 (2.9–5.6). The proportion of patients with FPG values above 15.0 mmol/l and requiring insulin therapy within 2 years was 60 and 15% for Ab+ve and Ab−ve patients respectively.
Kaplan–Meier plots of the proportion of patients randomised to conventional glucose control, or intensive glucose control with sulphonylurea, who became insulin-requiring according to clinical criteria during the first 10 years from diagnosis of diabetes. a Patients with FPG, after the dietary run-in, of 6.0–14.9 mmol/l. b Patients with FPG, after the dietary run-in, of 15.0 mmol/l or more. Conventional Ab−ve (solid line); conventional Ab+ve (short dashes); sulphonylurea Ab−ve (long dashes); sulphonylurea Ab+ve (dashes and dots)
Discussion
Patients diagnosed clinically with type 2 diabetes but with islet autoantibodies have an increased likelihood of early progression to insulin treatment [16, 17]. This was confirmed in a previous UKPDS report, which reviewed the 6-year follow-up of 1,870 patients who had not been assigned to insulin therapy [3]. The present study substantially extends these findings by analysing data from a larger cohort of UKPDS patients (4,545 of 5,102), including those who were allocated to insulin, and by examining glycaemic responses to randomised therapy over a 10-year period from diagnosis of diabetes. ICA, GADA and IA-2A measurements for this study were made with validated assays [10, 11, 18].
The one in eight UKPDS patients who were Ab+ve had a higher HbA1c value after the dietary run-in than the Ab−ve patients. Autoantibody status, however, did not differentially influence the glycaemic response to randomised allocation to diet, sulphonylurea or insulin therapy over the first 10 years from diagnosis in those patients achieving an FPG value, after the dietary run-in, of 6.0–14.9 mmol/l with no hyperglycaemic symptoms. Similarly in those with an FPG value after the dietary run-in of 15.0 mmol/l or more, or hyperglycaemic symptoms, the autoantibody status did not influence the HbA1c response to randomised allocation to sulphonylurea or insulin. In both groups, Ab+ve compared with Ab−ve patients became insulin-requiring more rapidly, regardless of whether they were allocated to dietary or sulphonylurea therapy. This in turn may have contributed to the greater weight gain seen in the Ab+ve patients.
Compared with Ab−ve patients, Ab+ve subjects were on average 4 years younger at diagnosis. Following the dietary run-in period, they were 6 kg leaner and had a 0.5% higher median HbA1c value, lower beta cell function and higher insulin sensitivity. They were also 2.6 times more likely to fail to achieve FPG values, after the dietary run-in, of less than 15.0 mmol/l. These findings are consistent with those from the smaller UKPDS sample reported previously, which identified age, BMI, antibody status and beta cell function as important predictors of progression to insulin therapy in patients with clinically diagnosed type 2 diabetes [3], as well as with those reported by Hosszufalusi et al [19].
The need to strive for good glycaemic control in type 2 diabetes has been confirmed by the UKPDS, which showed that more intensive blood glucose control reduced the risk of microvascular disease [6]. In addition, epidemiological analyses of UKPDS data demonstrated a continuous positive relationship between HbA1c and the risk of microvascular and macrovascular complications [20]. The similar HbA1c changes over time in response to different randomised therapies, irrespective of autoantibody status, demonstrate that sulphonylurea therapy remains a viable initial option in patients achieving FPG values lower than 15.0 mmol/l in response to initial dietary intervention, provided that regular monitoring of glycaemic control guides the need to introduce insulin therapy. Autoantibody status is informative, particularly in patients who cannot achieve FPG values of less than 15.0 mmol/l with dietary intervention, since 60% of these Ab+ve patients became insulin-requiring within 2 years of diagnosis when allocated to sulphonylurea therapy.
There is evidence from UKPDS data [3] and other studies [21] that the combination of ICA and GAD autoantibodies is a stronger indicator of insulin deficiency than the presence of either autoantibody alone, especially in patients over the age of 45 years [3]. High-titre GADA may also be predictive of early insulin requirement [21, 22]. In a separate UKPDS report [23], knowledge of IA-2A status did not improve prediction of early insulin use relative to predictability when ICA and GADA assay results were available. In the patients studied here, those with dual or triple autoantibody positivity who failed to achieve FPG values, after the dietary run-in, of less than 15.0 mmol/l had similar median FPG and HbA1c profiles over 10 years, irrespective of whether they were randomised to sulphonylurea or to insulin therapy (data not shown).
Systolic blood pressure, LDL-cholesterol and HDL-cholesterol are independent modifiable vascular risk factors in type 2 diabetes [24]. Cross-sectional studies have shown that Ab+ve patients have a lower systolic blood pressure than Ab−ve subjects [16, 25]. This may, as in the present study, reflect a concomitantly lower BMI, since systolic blood pressure was only lower in our more hyperglycaemic Ab+ve patients. A higher plasma LDL-cholesterol has also been reported [16], but this was in a sample of 17 ICA-positive patients and has not been confirmed in other studies [22, 25] or in the present larger patient cohort. A higher plasma HDL-cholesterol [16, 25] and lower plasma triglycerides [22, 25] have been associated with autoantibody positivity as shown here. The contribution of plasma triglyceride to vascular risk in diabetes is, however, uncertain [24]. Despite their higher HbA1c, fewer Ab+ve patients met the NCEP ATPIII criteria for the metabolic syndrome, and this was consistent with their younger age, leaner body mass, lower blood pressure and lower total to HDL-cholesterol ratio relative to Ab−ve patients. This difference was observed also in those patients who were more hyperglycaemic initially.
The present study has confirmed that positivity for diabetes-related islet autoantibodies can be found in a significant proportion of younger, newly diagnosed patients with clinically defined type 2 diabetes. Although patients with diabetes-related islet autoantibodies can be treated initially with sulphonylurea, provided that glycaemic control is monitored carefully, they are more likely to require insulin at an earlier stage than antibody-negative patients.
Abbreviations
- Ab+ve:
-
Autoantibody-positive
- Ab−ve:
-
Autoantibody-negative
- FPG:
-
Fasting plasma glucose
- GADA:
-
Glutamic acid decarboxylase autoantibodies
- HOMA:
-
Homeostasis model assessment
- IA-2A:
-
Islet autoantibody 2A
- ICA:
-
Islet cell autoantibodies
- IQR:
-
Interquartile range
- JDF:
-
Juvenile Diabetes Federation
- NCEP ATP III:
-
National Cholesterol Education Program Adult Treatment Program III
- NHS:
-
National Health Service
- RR:
-
Relative risk
- UKPDS:
-
United Kingdom Prospective Diabetes Study
References
Tuomi T, Groop LC, Zimmet PZ, Rowley MJ, Knowles W, Mackay IR (1993) Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes 42:359–362
Niskanen LK, Tuomi T, Karjalainan J, Groop LC, Uusitupa MIJ (1995) GAD antibodies in NIDDM. Diabetes Care 18:1557–1565
UKPDS Group (1997) UKPDS 25: clinical value of ICA and GADA in predicting insulin requirement in patients with newly diagnosed NIDDM at different ages. Lancet 350:1288–1293
Rosenbloom AL (2003) Obesity, insulin resistance, b-cell autoimmunity, and the changing clinical epidemiology of childhood diabetes. Diabetes Care 26:2954–2956
UKPDS Group (1991) UK prospective diabetes study VIII: study design, progress and performance. Diabetologia 34:877–890
UKPDS Group (1998) Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
UKPDS Group (1998) UK Prospective Diabetes Study 24: relative efficacy of sulfonylurea, insulin and metformin therapy in newly diagnosed non-insulin dependent diabetes with primary diet failure followed for six years. Ann Intern Med 128:165–175
Bonifacio E, Bingley PJ, Shattock M et al (1990) Quantification of islet-cell antibodies and prediction of insulin dependent diabetes. Lancet 147–149
Verge CF, Stenger D, Bonifacio E et al (1998) Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: combinatorial islet autoantibody workshop. Diabetes 47:1857–1866
Bonifacio E, Lampaspona V, Genovese S, Ferrari M, Bosi E (1995) Identification of protein tyrosine phosphatase-like IA2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K autoantigen and a component of islet cell antibodies. J Immunol 155:5419–5426
Bonifacio E, Lampaspona V, Bingley PJ (1998) IA-2 (islet cell antigen 512) is the primary target of humoral autoimmunity against Type 1 diabetes-associated tyrosine phosphate autoantigens. J Immunol 161:2648–2654
UKPDS Group (1994) UK prospective diabetes study XI: biochemical risk factors in type 2 diabetic patients at diagnosis compared with age-matched normal subjects. Diabet Med 11:534–544
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Levy J, Matthews D, Hermans M (1998) Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21:2191–2192
Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of the national cholesterol education program (NCEP). JAMA 285:2486–2497
Gottsater A, Ahmed M, Lilja B, Fernlund P, Sundkvist G (1996) Islet cell antibodies at diagnosis, but not leanness, relate to a better cardiovascular risk factor profile 5 years after diagnosis of NIDDM. Diabetes Care 19:60–63
Willis JA, Scott RS, Brown LJ et al (1996) Antibodies against glutamic acid decarboxylase in newly diagnosed adult-onset diabetes mellitus. Diabetes Res Clin Pract 33:89–97
Bingley PJ, Bonifacio E, Mueller P (2003) Diabetes antibody standardization program: first assay proficiency evaluation. Diabetes 52:1128–1136
Hosszufalusi N, Vatay A, Rajczy K et al (2004) Similar genetic features and different islet cell autoantibodies in adults (LADA) compared with adult-onset type 1 diabetes with rapid progression. Diabetes Care 26:452–457
UKPDS Group (2000) Association of glycaemia with macrovascular and microvascular complications of Type 2 diabetes (UKPDS 35). BMJ 321:405–412
Lohmann T, Kellner K, Verlohren HJ et al (2001) Titre and combination of ICA autoantibodies to glutamic acid and decarboxylase discriminate two clinically distinct types of latent autoimmune diabetes in adults (LADA). Diabetologia 44:1005–1010
Davis TME, Zimmett P, Davis WA, Fida S, Bruce D, Mackay I (2000) Autoantibodies to glutamic acid decarboxylase in diabetic patients from a multi-ethnic Australian community. Diabet Med 17:667–674
Bosi E, Cull CA, Bonifacio E et al (2004) IA-2 antibody prevalence and risk assessment of early insulin requirement in subjects presenting as type 2 diabetes. Diabetologia DOI:10.1007/s00125-005-1691-9
UKPDS Group (1998) Risk factors for coronary artery disease in non-insulin dependent diabetes (UKPDS 23). BMJ 316:823–828
Tuomi T, Carlsson A, Isomaa B et al (1999) Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes 48:150–157
Acknowledgements
The cooperation of the patients and many National Health Service (NHS) and non-NHS staff at the centres is much appreciated. The major grants for this study were from the UK Medical Research Council, British Diabetic Association, The British Heart Foundation, The UK Department of Health, The Italian Ministry of Health, The National Eye Institute and The National Institute of Digestive, Diabetes and Kidney Disease in the National Institutes of Health USA, Novo-Nordisk, Bayer, Bristol Myers Squibb, Hoechst, Lilly, Lipha and Farmitalia Carlo Erba. Other funding companies and agencies, the supervising committees and all participating staff are acknowledged in an earlier paper [6]. We are also grateful to the Wellcome Trust for a grant for the measurement of ICA and GADA. We thank Marion Shattock for the ICA assay, Dr Merrill Rowley for supervision, and Ray Spark for the technical performance of GADA assays in the Monash University Laboratory. We thank Dr Mattia Locatelli for helpful review of the manuscript.
Participating UK Centres
Radcliffe Infirmary, Oxford; Royal Infirmary, Aberdeen; University Hospital, Birmingham; St. George’s Hospital, Hammersmith Hospital and Whittington Hospital, London; City Hospital and Royal Victoria Hospital, Belfast; North Staffordshire Royal Infirmary, Stoke-on-Trent; St. Helier Hospital, Carshalton; Norfolk and Norwich Hospital, Norwich; Lister Hospital, Stevenage; Ipswich Hospital, Ipswich; Ninewells Hospital, Dundee; Northampton General Hospital, Northampton; Torbay Hospital, Torbay; Peterborough General Hospital, Peterborough; Scarborough Hospital, Scarborough; Derbyshire Royal Infirmary, Derby; Manchester Royal Infirmary, Manchester; Hope Hospital, Salford; Leicester General Hospital, Leicester; Royal Exeter & Devon Hospital, Exeter.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davis, T.M.E., Wright, A.D., Mehta, Z.M. et al. Islet autoantibodies in clinically diagnosed type 2 diabetes: prevalence and relationship with metabolic control (UKPDS 70). Diabetologia 48, 695–702 (2005). https://doi.org/10.1007/s00125-005-1690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1690-x