Abstract
Aims/hypothesis
The role of gamma-aminobutyric acid (GABA) and A-type GABA receptors (GABAARs) in modulating islet endocrine function has been actively investigated since the identification of GABA and GABAARs in the pancreatic islets. However, the reported effects of GABAAR activation on insulin secretion from islet beta cells have been controversial.
Methods
This study examined the hypothesis that the effect of GABA on beta cell insulin secretion is dependent on glucose concentration.
Results
Perforated patch-clamp recordings in INS-1 cells demonstrated that GABA, at concentrations ranging from 1 to 1,000 μmol/l, induced a transmembrane current (IGABA) which was sensitive to the GABAAR antagonist bicuculline. The current–voltage relationship revealed that IGABA reversed at −42±2.2 mV, independently of glucose concentration. Nevertheless, the glucose concentration critically controlled the membrane potential (V M), i.e., at low glucose (0 or 2.8 mmol/l) the endogenous V M of INS-1 cells was below the IGABA reversal potential and at high glucose (16.7 or 28 mmol/l), the endogenous V M of INS-1 cells was above the IGABA reversal potential. Therefore, GABA dose-dependently induced membrane depolarisation at a low glucose concentration, but hyperpolarisation at a high glucose concentration. Consistent with electrophysiological findings, insulin secretion assays demonstrated that at 2.8 mmol/l glucose, GABA increased insulin secretion in a dose-dependent fashion (p<0.05, n=7). This enhancement was blocked by bicuculline (p<0.05, n=4). In contrast, in the presence of 28 mmol/l glucose, GABA suppressed the secretion of insulin (p<0.05, n=5).
Conclusions/interpretation
These findings indicate that activation of GABAARs in beta cells regulates insulin secretion in concert with changes in glucose levels.
Similar content being viewed by others
Introduction
Gamma-aminobutyric acid (GABA), which is synthesised from glutamic acid by the rate-limiting enzyme GAD, is a major inhibitory neurotransmitter in the mammalian central nervous system [1]. GABA induces rapid inhibition primarily by activating the A-type GABA receptor (GABAAR) [2], a heteropentameric Cl– ion channels complex [3]. In most cases, activation of GABAAR in neurons results in membrane hyperpolarisation as a consequence of an inward Cl– flux [3].
The concentrations of GABA and GAD and the density of GABAAR in pancreatic islets are comparable with those encountered in the central nervous system [4–8], suggesting that the GABA–GABAAR system plays a role in modulating islet endocrine functions. In the pancreas, GABA is primarily produced by insulin-secreting beta cells [9–11] and probably by the glucagon-containing alpha cells [9, 12]. GABAARs are expressed in both beta and alpha cells [13–15], suggesting that GABA modulates pancreatic endocrine function in an autocrine and paracrine manner [16–18]. In pancreatic beta cells, GABA is associated with synaptic-like microvesicles that are distinct from the insulin-containing large dense-core vesicles [10, 11]. Release of GABA from beta cells appears to be regulated by nutrients [19] including glucose [19–21].
The effect of GABA on insulin secretion from the beta cells has also been controversial. For example, perfusion studies using rat and dog pancreas have shown that GABA inhibits arginine-stimulated insulin release [16, 21]. Other studies demonstrate that both GABA and GABAAR agonists have no detectable effect on insulin secretion in perfused rat pancreas [22, 23]. However, GABA-induced increases in insulin release were observed in rat pancreas and isolated human insulinoma cells [13, 24, 25].
The direction of the GABAAR-mediated Cl− current depends on membrane potential (V M), which, in pancreatic beta cells, is critically regulated by extracellular glucose [26, 27]. We hypothesise that the effect of GABA on insulin secretion from beta cells is dependent on the extracellular glucose concentration. Using perforated patch-clamp recordings and insulin secretion assays, we examined in insulin-secreting INS-1 cells the effects of GABA on V M and insulin secretion in the presence of different levels of glucose. Our results indicate that, depending on the extracellular glucose levels, GABA can exert either excitatory or inhibitory effects on beta cells.
Materials and methods
Cell culture
INS-1 cells (passage 50–65) were maintained in RPMI 1640 medium (Invitrogen, Burlington, ON, Canada) containing fetal bovine serum (10% v/v), 100 Units/ml penicillin G sodium, 100 μg/ml streptomycin sulphate, 55 mg/500 ml sodium pyruvate, 1.14 g/500 ml HEPES, and 1.7 μl/500 ml β-mercaptoethanol at 37°C in an atmosphere of humidified air (95%) and CO2 (5%). In studies involving serum-starvation, serum was replaced by 0.1% BSA in RPMI 1640 without glucose.
RT-PCR
Total cellular RNA was extracted from confluent 10-cm plates of INS-1 cells using Trizol (Invitrogen Life Technologies, CA, USA) following the manufacturer’s instructions. GABAAR mRNA transcripts were detected using a one-step RT-PCR kit (Qiagen). Briefly, 100 ng total RNA were used in 25-μl one-step RT-PCR reactions containing 0.4 mmol/l dNTPs and 0.6 μmol/l of each primer. The template was omitted for the negative control. Thermocycler conditions were 50°C for 30 min, 95°C for 15 min, and 35 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min. This was followed by a 10-min extension at 72°C. The RT-PCR products were separated on a 1% agarose gel and visualised with ethidium bromide. The GABAAR gene-specific primers used were as follows: α1 FP: cgggaagaagctatggaca; α1 RP: ccactcatagacaacttctgc; α2 FP: tctagacagacttctggatgg; α2 RP: agagtcagaagcattgtaagtc; α3 FP: cccagatattcctgacgatag; α3 RP: tgagccatcctgagctactt; β1 FP: cgactgctcaaaggatatgac; β1 RP: agtgatagtcgtggatatgcc; β2 FP: gctgtctgtgctcagagtgt; β2 RP: gtgactgcattgtcatcgcc; β3 FP: acggtcgacaagctgttgaa; β3 RP: tcgagcagcagatgcatc; γ1 FP: gtccagatataggcgtgaga; γ1 RP: ctccagtacttaggatcagc; γ2 FP: gcattggtccagtgaatgct; γ2 RP: ggaatgtaggtctggatggt; γ3 FP: acagcattggtcctgtgtca; γ3 RP: caaactgatagagccgccat.
Immunoblotting
Cells were lysed in buffer containing 1% Triton X-100 and protease and phosphatase inhibitors, as previously described [28]. Proteins (50 μg) were separated by 10% SDS-PAGE and electrotransferred onto a nitrocellulose filter (0.2 μm; Bio-Rad Laboratories, Hercules, CA, USA). After probing with mouse anti-beta2/3 isoforms of GABAAR IgG (1:1,000; Upstate Biotechnology, Lake Placid, NY, USA), the proteins were visualised with horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000; Upstate Biotechnology) using an electrochemiluminescence detection technique (Amersham, Piscataway, NJ, USA), as described previously [28].
Immunochemistry
Cells grown on glass coverslips in 12-well plates were fixed with paraformaldehyde (3.7% in PBS) and permeabilised with 0.2% Triton X-100. After incubation with 5% goat serum and 1% BSA (in PBS) for 60 min, cells were incubated with mouse antibodies against beta2/3 GABAAR subunits (1:100; Upstate Biotechnology) overnight at 4°C. After washing, cells were incubated with Cy3-conjugated goat anti-mouse IgG (1:500, 30 min; Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Images were visualised using a Leica TCS 4D laser confocal fluorescence microscope.
Electrophysiology
Perforated patch recordings were performed at room temperature (23–25°C). One hour before recording, cells were bathed in the standard glucose-free extracellular solution containing (in mmol/l) 145 NaCl, 1.3 CaCl2, 5.4 KCl, 25 HEPES, (pH 7.4). Recordings were performed using an Axopatch-1D amplifier (Axon Instruments, Foster City, CA, USA). Electrodes (3–5 MΩ) were constructed from thin-walled glass (1.5 mm diameter; World Precision Instruments, Sarasota, FL, USA) using a two-stage puller (PP-830; Narishige, East Meadow, NY, USA). The standard intracellular solution for perforated patch recording consisted of (in mmol/l) 150 K-gluconate, 10 KCl, 10 KOH, 10 HEPES, 2 MgCl2 and 1 CaCl2. After addition of the pore-forming agent amphotericin B (Sigma-Aldrich Corp., Buchs, Switzerland) [27], the pH of the intracellular solution was adjusted to 7.30 (with KOH) and the osmolarity was corrected to a range of 310–315 mOsm.
Perforated recordings started under voltage-clamp mode. The effect of amphotericin B was observed as a constant decrease in serial resistance after the electrode seal. In most of the recordings, the resistance declined to a value ranging from 28 to 30 MΩ within 5–15 min after the seal, and then stabilised for 45–60 min. All perforated patch recordings began when the serial resistance had attained values below 28 MΩ. To monitor a possible formation of whole-cell configuration, a testing voltage-ramp (a gradual voltage change from –80 to 60 mV in 1.5 s) was applied to the cell at the start of recording, and approximately every 10 min during the recording. With this testing protocol, a sigmoid-shaped current–voltage (I–V) curve was seen under stable perforated patch recordings, whereas a linear I–V relationship gradually appeared after whole-cell configuration. If a sudden change in the I–V relationship occurred, the recording was not used for the study. To measure the GABAAR-channel activity in INS-1 cells, perforated patch recordings were carried out under voltage-clamp mode and cells were held at –60 mV unless otherwise indicated. Different concentrations of GABA (1.0–1000 μmol/l) were focally applied to the recorded cells by means of a computer-controlled multi-barrelled perfusion system (SF-77B; Warner Instruments, Hamden, CT, USA). To investigate the effect of GABA on the excitability of INS-1 cells in different concentrations of glucose, changes in the V M were evaluated via current-clamp recordings. The electrical signals were digitised, filtered (1 kHz), and acquired on-line using the program Clampex and analysed off-line by means of the program Clampfit (Axon Instruments).
Intracellular Ca2+ measurement
Intracellular Ca2+ concentration ([Ca2+]i) was measured using Fura 2-AM (Molecular Probes). Cells grown on coverslips (70–80% confluency) were loaded with Fura-2 AM (3 μmol/l) in a medium containing (in mmol/l)130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 NaHCO3 and 10 HEPES, pH 7.4 for 50 min at 37°C. Cells were washed and transferred to an open chamber equipped with a TC-324B Heater Controller (Warner Instruments) and perfused with the same medium at 1 ml/min (36–37°C) while the recordings were made using an Olympus BX51W1 fluorescent microscope. Cells were excited by dual excitation at 340/380 nm using a DeltaRam high-speed monochromator (Photon Technology International) and the emissions were detected at 510 nm by a band pass filter. The system was equipped with ImageMaster 3 software for control of the monochromator, the video camera, and collecting data.
Insulin secretion
Insulin concentrations were measured using a rat insulin RIA kit (Linco Research, St. Louis, MO, USA) as previous described [28]. INS-1 cells grown in 24-well plates to 85–90% confluency were rinsed twice and incubated with KRB (containing, in mmol/l, 115 NaCl, 5 KCl, 24 NaHCO3, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 0.1% BSA) for 60 min. Cells were incubated in KRB in the presence of various concentrations of glucose (dextrose; Merck KgaA, Darmstadt, Germany) and/or GABA (Sigma, St. Louis, MO, USA) as indicated. After 30 min of incubation, the media were collected and insulin levels measured by RIA using the rat insulin RIA kit according to the manufacturer’s instructions. The insulin secretion was normalised to the cellular protein content. Protein was determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, CA, USA).
Statistical analysis
Data are expressed as means±SEM. For insulin secretion assays, the number of samples and separate experiments are described in the figure legends. Statistical difference between groups was analysed by ANOVA followed by a Student–Newman–Keuls test to estimate the differences between each pair of groups when applicable. For electrophysiological analysis, data were compared with Student’s unpaired or paired t-test where appropriate. A p value <0.05 was considered as significant. Statistical analyses were performed using SigmaStat from Jandel Scientific Software (San Rafael, CA, USA).
Results
Expression of GABAA receptor in INS-1 cells
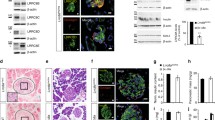
RT-PCR experiments demonstrated that INS-1 cells express transcripts of GABAAR subunits alpha 1, alpha 2, alpha 3, beta 3 and gamma 3 (Fig. 1a). It is known that in neurons, a functional GABAAR is usually a pentamer composed of two alpha, two beta and one gamma receptor subunits [2]. Thus, INS-1 cells appear to possess the necessary subunits for a functional GABAAR. Furthermore, Western blot analysis using a specific antibody directed against the beta2/3 subunits of GABAAR detected the expression of GABAAR proteins in INS-1 cells (Fig. 1b). Expression and membrane localisation of GABAAR were also confirmed by immunostaining and confocal imaging studies (Fig. 1c). In addition, immunostaining of rat pancreas sections using anti-alpha1 and beta2/3 subunit antibodies showed that islet beta cells express alpha1 and beta2 or beta 3 subunits (data not shown).
Expression of GABAA receptors in INS-1 cells. a RT-PCR to detect different GABAAR subunit nucleotide sequences (See Materials and methods). Total RNA from INS-1 cells were used in one-step RT-PCR reactions using specific primers for the alpha1–3, beta1–3, and gamma1–3 subunits to detect expression of the different subunits. In the control reaction, the template was omitted. b Western blot on INS-1 cell lysates (50-μg protein) using a specific antibody against the beta2/3 subunits of the GABAAR. COS cell lysates were used as control. c Immunocytochemistry of INS-1 cells using the antibody against beta2/3 subunits of the GABAAR. COS cells were used in the control staining
GABAARs in INS-1 cells are functional
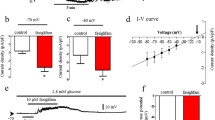
To investigate whether the GABAARs in INS-1 cells were functional, we applied different concentrations of GABA to the cells by means of a fast perfusion system, and measured transmembrane currents using voltage-clamp recording techniques (Fig. 2a). We found that when held at −60 mV, about 60% of cells generated visible inward currents in response to 1 μmol/l GABA. Yet, currents were generated in all tested cells when GABA concentrations were higher than 3 μmol/l. The GABA-induced currents were concentration-dependent (Fig. 2a,b), with an EC50 of 22.3 μmol/l (Fig. 2b). We noticed that at lower concentrations of GABA (<30 μmol/l), the amplitude of the GABA-evoked current (IGABA) was stable (not shown). However, at higher concentrations (>100 μmol/l), IGABA exhibited a rapid onset, characterised by a peak, followed by a gradual decay to a steady state (Fig. 2c, left). In response to 100 μmol/l GABA, the peak amplitude of the IGABA in individual cells varied from 12 to 470 pA (Fig. 2d) (control IGABA: 117±33 pA, n=15). Notably, IGABA could be reversibly suppressed by co-application of the competitive GABAAR antagonist, bicuculline (50 μmol/l; bicuculline IGABA: 7±2 pA, n=4; p<0.05) (Fig. 2d). These results demonstrate that the GABAARs in INS-1 cells are physiologically functional.
Transmembrane current evoked by GABA. a GABA-evoked currents in INS-1 cells via activation of GABAARs. In a perforated voltage-clamp mode, when cells were voltage clamped at −60 mV, fast application of GABA (b) dose-dependently evoked inward currents (n≥8 cells at each testing concentration of GABA). c The effect of GABAAR antagonist bicuculline (Bic, 50 μmol/l) on GABA (100 μmol/l)-induced current. The peak amplitude (d) of GABA currents in the absence (Ctrl) and presence of bicuculline was plotted (Ctrl: 117±33 pA, n=15; Bic: 7±2 pA, n=4; p<0.05). e In the absence of glucose, GABA (100 μmol/l) evoked inward and outward currents in the same INS-1 cell when held at different membrane potentials. f The reversal potential for GABA currents in the absence of glucose was determined by analysing the current–voltage relationship (−42±2.2 mV, n=6 cells). g In the presence of 16.7-mmol/l glucose, GABA currents were reversed at −43 mV
We further characterised the I–V relationship of GABAAR-gated channels in INS-1 cells. GABA (100 μmol/l) was applied to cells held at different V M levels (–80 to 40 mV, see Fig. 2e) and the current was recorded. The plotted I–V curve indicated that in the absence of extracellular glucose, I GABA reversed at –42±2.2 mV (Fig. 2f, n=6). Glucose is one of the key physiological regulators of beta cell functions. However, the reversal potential of IGABA in INS-1 cells was not affected by addition of 16.7 mmol/l glucose to the extracellular solution (Fig. 2g), suggesting that glucose concentration does not affect this property of GABAARs, or the equilibrium potential of Cl– in INS-1 cells.
GABA regulates the excitability of INS-1 cells differently under different glucose concentrations
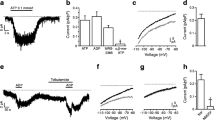
Glucose concentrations dictate the ratio of intracellular ATP/ADP concentrations, which in turn regulate the activity of ATP-sensitive K+ (KATP) channels, thereby controlling the V M of beta cells [27, 29–31]. We tested the hypothesis that the level of glucose sets the instantaneous V M, which in turn determines the effect of GABAAR activation on the excitability of beta cells. Using current-clamp recordings, we measured the change in V M of INS-1 cells in response to increases of glucose concentration (0 to 2.8, 16.7 or 28 mmol/l, respectively, Fig. 3a). In the absence of glucose, V M of INS-1 cells varied from −51 to −82 mV (Fig. 3b: −65±3 mV, n=12). Increasing glucose concentrations depolarised the membrane of INS-1 cells in a concentration-dependent manner (Fig. 3a,b: V M at 2.8 mmol/l glucose: −53±2 mV; V M at 16.7 mmol/l: −32±2 mV; V M at 28 mmol/l: −22±2 mV, n=6). In some cases, bursts of action potentials were seen during high-glucose-induced depolarisation (Fig. 3a).
Regulation of beta cell excitability by GABA in the presence of different concentrations of glucose. a Increase in glucose concentrations (G) from 0 mmol/l to 2.8, 16.7 and 28 mmol/l, caused a dose-dependent depolarisation of the cell (traces at 2.8, 16.7 and 28 G were from the same cell). Note that bursting of action potentials was generated in some cells in the presence of high glucose (c). b The membrane potentials (VM) of INS-1 cells in the presence of various concentrations of glucose are summarised (at 0 mmol/l: −65±3 mV, n=12; at 2.8 mmol/l: −53±2 mV, n=6; at 16.7 mmol/l: −32±2 mV, n=7; at 28 mmol/l: −22±2 mV, n=6). c Example traces illustrate the dose-dependent effect of GABA on V M in the absence (0 G), and presence of 28 mmol/l (28 G) of glucose. The grey bars under or above each trace indicate the duration of GABA perfusion. d, e Summarised data reveal the concentration-dependency of GABA on V M in 0 mmol/l and 28 mmol/l of glucose, respectively. f Plotted graph indicates the direction and the scope of GABA-induced changes in V M at different glucose concentrations (0 mmol/l: 10±1.2 mV, n=11; 2.8 mmol/l: 6.5±0.7 mV, n=5; 16.7 mmol/l: −8.5±5 mV, n=5; 28 mmol/l: −16.0±5 mV, n=5)
Next, we examined the effect of different concentrations of GABA on V M at low and high concentrations of glucose (Fig. 3c). GABA (1–1,000 μmol/l) induced membrane depolarisation as a function of dose in the absence of glucose, but caused membrane hyperpolarisation in the presence of 28 mmol/l glucose (Fig. 3c). The EC50 value of GABA on V M at low glucose was similar to that at high glucose (EC50 at 0 mmol/l glucose: 24.4; EC50 at 28 mmol/l glucose: 20.8 μmol/l, Fig. 3d,e), suggesting that glucose does not affect the affinity of GABA for GABAARs. We further examined GABA (100 μmol/l)-induced changes in V M at 0, 2.8, 16.8 and 28 mmol/l glucose, and confirmed that GABA could up- and downregulate the V M of beta cells in accordance with glucose concentrations (Fig. 3f, at 0 mmol/l glucose: depolarisation 12±1.2 mV, n=11; at 2.8 mmol/l: depolarisation 6.5±0.7 mV, n=9; at 16.8 mmol/l: hyperpolarisation –8.5±5 mV, n=9; at 28 mmol/l: hyperpolarisation –16.0±5 mV, n=9).
GABA increases the cytosolic calcium concentration of INS-1 cells
Using fluorescent probe, Fura 2-AM, we measured [Ca2+]i of individual INS-1 cells in response to GABA treatment. Perfusion of GABA (100 μmol/l) at low glucose (2.8 mmol/l) led to a significant increase of [Ca2+]i (Fig. 4a,b: representative [Ca2+]i of two individual cells). Presumably, this fact reflects depolarisation of the cells by GABA, because membrane depolarisation induced by increasing extracellular KCl (to 50 mmol/l) also drastically elevated [Ca2+]i of the INS-1 cells. In all cells tested, the increase of [Ca2+]i during GABA treatment displayed oscillatory behaviour (Fig. 4a,b, n=3; 30–50 cells were measured from each experiment). Perfusion of 28 mmol/l glucose led to a drastic increase in cytosolic Ca2+ oscillation (Fig. 4c,d). In this case, application of GABA (100μmol/l) suppressed the amplitude of Ca2+ oscillation spikes (Fig. 4c,d). Nevertheless, an increase in the frequency of Ca2+ oscillation was observed in some cells (Fig. 4c).
Modulation of Ca2+ influx and insulin secretion by GABA in INS-1 cells. a–d Effect of GABA (100 μmol/l) on [Ca2+]i in INS-1 cells. Cells grown on coverslips were loaded with Fura-2 AM and transferred to a chamber and perfused at 1 ml/min (36–37°C) as described in Materials and methods. Horizontal lines show perfusion with corresponding reagents; the glucose (G) levels are outlined by the grey lines. The typical time-dependent [Ca2+]i response from individual cells is shown and represents three independent experiments in which 30–50 cells were measured from each experiment at low glucose (a, b, 2.8 mmol/l) or high glucose (c, d, 28 mmol/l). e Glucose-stimulated insulin secretion is concentration-dependent (n=4). Cells were incubated in KRB in the presence of various concentrations of glucose (Glu) as indicated. After 30 min of incubation, the media were collected and insulin levels were measured by RIA. f In the presence of 2.8 mmol/l glucose, insulin secretion was measured in the presence of different concentrations of GABA (n=7), with or without GABAAR antagonist bicuculline (Bic, 100 μmol/l, n=4). g Effect of GABA (100 μmol/l) and bicuculline (Bic, 100 μmol/l) on insulin secretion at 28 mmol/l glucose (Glu, n=4). *p<0.05, **p<0.01, ***p<0.005
GABA-modulated insulin secretion in INS-1 cells is dependent on glucose concentration
Next, we determined whether GABA affects insulin secretion in INS-1 cells using insulin secretion assays. Glucose stimulated insulin secretion from INS-1 cells in a dose-dependent manner (Fig. 4e). Consistent with the electrophysiological data, under low glucose conditions (2.8 mmol/l), GABA (1–1,000 μmol/l) steadily increased insulin secretion (Fig. 4f, p<0.05, n=7). This effect of GABA was significantly diminished by the GABAAR antagonist bicuculline (Fig. 4f, n=4). In contrast, under high glucose conditions (28 mmol/l), GABA significantly suppressed insulin secretion (Fig. 4g, p<0.05, n=5). This suppression was also significantly attenuated by co-incubation of the cells with bicuculline (Fig. 4g, p<0.05, n=4). Bicuculline, by itself, had no significant effect on insulin secretion under low and high glucose conditions (Fig. 4f,g, p>0.05, n=4). In contrast, the GABABR agonist, baclofen had no significant effect on GABA-modulated insulin secretion from INS-1 cells at either low (2.8 mmol/l) or high (28 mmol/l) glucose (2.8 mmol/l: control vs. baclofen=1.0±0.1 vs. 0.98±0.09; 28 mmol/l: control vs. baclofen=1±0.16 vs. 1.12±0.19; p>0.05, n=3). These results indicate that activation of GABAAR up- and downregulates insulin secretion depending on the extracellular concentration of glucose.
Discussion
Previous studies [4–8] suggest that the pancreatic GABA–GABAAR system plays a role in modulating islet endocrine functions. However, the reported effects of GABA on insulin secretion from the beta cells have been controversial. In this study we demonstrated a mechanism by which GABA modulates insulin secretion from beta cells in concert with changes in glucose concentration.
We found that GABAARs are expressed in the widely used beta cell line INS-1 cells (Fig. 1), which is consistent with previously reported studies in isolated human insulinoma cells [13] and rat pancreatic beta cells [15]. Since GABA is localised mainly within the pancreatic beta cells [32], identification of GABAARs in the beta cells suggests that the GABA–GABAAR system may serve as both an autocrine and paracrine modulator in the islets. We also demonstrated that, dependent on dose, perfusion of GABA induced transmembrane currents in INS-1 cells, and that the GABA-induced current was sensitive to the GABAAR antagonist, bicuculline (Fig. 2a,c). These results indicate that GABAARs in INS-1 cells are functional.
It is well known that by modulating the KATP conductance, glucose concentration critically sets the instantaneous V M of beta cells [27]. We confirmed that with increasing glucose concentrations, from 0 to 28 mmol/l, the V M of INS-1 cells changed from approximately −65 mV to approximately −22 mV. On the other hand, we found that changing glucose concentrations did not affect the reversal potential of GABAAR-mediated currents, which was at about −42 mV in INS-1 cells. Therefore, activation of GABAAR could potentially up- and downregulate the excitability of the cell, in accordance with changes in glucose concentrations. Indeed, our results demonstrated that while the cell membrane hyperpolarised in the presence of low glucose, application of GABA positively shifted the V M. In contrast, under conditions of high glucose, GABA negatively shifted the elevated V M. These data suggest that GABA, in concert with glucose, plays a role in regulation of excitability of the beta cell, thus regulating insulin secretion. This notion was strongly supported by the findings that GABA elevated the [Ca2+]i. oscillations at low glucose (Fig. 4a), but suppressed the amplitude of high-glucose-induced Ca2+-oscillation spikes (Fig. 4c).
Our insulin RIA results further confirmed that GABA stimulated insulin secretion at low (2.8 mmol/l) glucose, but inhibited insulin secretion at high (28 mmol/l) glucose. The bi-directional regulation of insulin secretion in beta cells by GABA at different glucose levels implies an important role for GABA in modulating islet hormonal secretion. GABA, by modulating insulin secretion from beta cells in concert with changes in glucose concentration, provides a unique mechanism for pancreatic endocrine regulation. Notably, in the presence of low glucose (2.8 mmol/l), GABA caused a moderate but significant increase in insulin secretion (∼20–40%, equivalent to ∼0.3–0.4 nmol/l). Intracellular Ca2+ measurements suggested that GABA-modulated insulin secretion in INS-1 cells may be via a mechanism involving Ca2+ movement.
Our findings in this study suggest that the GABA system may function as a negative feedback regulating mechanism in the islets. Under low glucose conditions, GABA release from beta cells increases [33, 34], which in turn may enhance insulin secretion. It has been demonstrated that insulin is a physiological inhibitor of glucagon release within islets [35] under in vitro [36, 37] and in vivo [38] conditions. Studies on islet microvasculature have shown that the alpha cells lie downstream from the beta cells, thus insulin released from beta cells can immediately act on alpha cells [39]. Therefore, physiologically, a modest change in insulin concentrations in the islet could have a significant impact on glucagon secretion from alpha cells. For instance, when hypoglycaemia occurs, GABA-stimulated insulin may act on the alpha cells to prevent the potential hyperglycaemia resulting from excessive glucagon secretion, because glucagon is a prominent facilitator of glucose production [40, 41]. Likewise, during hyperglycaemia, GABA depresses insulin secretion thus preventing a potential hypoglycaemia resulting from insulin ‘overshooting’.
Abbreviations
- GABAAR:
-
A-type GABA receptor
- [Ca2+]i :
-
intracellular Ca2+ concentration
- I–V :
-
current voltage
- GABA:
-
gamma-aminobutyric acid
- V M :
-
membrane potential
- IGABA :
-
GABA-induced transmembrane current
References
Gottlieb DI (1988) GABAergic neurons. Sc Am 258:82–89
Kittler JT, McAinsh K, Moss SJ (2002) Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol 26:251–268
Kittler JT, Moss SJ (2003) Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol 13:341–347
Briel G, Gylfe E, Hellman B, Neuhoff V (1972) Microdetermination of free amino acids in pancreatic islets isolated from obese-hyperglycemic mice. Acta Physiol Scand 84:247–253
Garry DJ, Coulter HD, McIntee TJ, Wu JY, Sorenson RL (1987) Immunoreactive GABA transaminase within the pancreatic islet is localized in mitochondria of the B-cell. J Histochem Cytochem 35:831–836
Michalik M, Erecinska M (1992) GABA in pancreatic islets: metabolism and function. Biochem Pharmacol 44:1–9
Okada Y, Taniguchi H, Schimada C (1976) High concentration of GABA and high glutamate decarboxylase activity in rat pancreatic islets and human insulinoma. Science 194:620–622
Michalik M, Nelson J, Erecinska M (1993) GABA production in rat islets of Langerhans. Diabetes 42:1506–1513
Chessler SD, Simonson WT, Sweet IR, Hammerle LP (2002) Expression of the vesicular inhibitory amino acid transporter in pancreatic islet cells: distribution of the transporter within rat islets. Diabetes 51:1763–1771
Thomas-Reetz A, Hell JW, During MJ, Walch-Solimena C, Jahn R, De Camilli P (1993) A gamma-aminobutyric acid transporter driven by a proton pump is present in synaptic-like microvesicles of pancreatic beta cells. Proc Natl Acad Sci USA 90:5317–5321
Sorenson RL, Garry DG, Brelje TC (1991) Structural and functional considerations of GABA in islets of Langerhans. Beta-cells and nerves. Diabetes 40:1365–1374
Gilon P, Campistron G, Geffard M, Remacle C (1988) Immunocytochemical localisation of GABA in endocrine cells of the rat entero-pancreatic system. Biol Cell 62:265–273
Glassmeier G, Hopfner M, Buhr H et al (1998) Expression of functional GABAA receptors in isolated human insulinoma cells. Ann NY Acad Sci 859:241–248
Rorsman P, Berggren PO, Bokvist K et al (1989) Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 341:233–236
Von Blankenfeld G, Turner J, Ahnert-Hilger G et al (1995) Expression of functional GABAA receptors in neuroendocrine gastropancreatic cells. Pflugers Arch 430:381–388
Gu XH, Kurose T, Kato S et al (1993) Suppressive effect of GABA on insulin secretion from the pancreatic beta-cells in the rat. Life Sci 52:687–694
Satin LS, Kinard TA (1998) Neurotransmitters and their receptors in the islets of Langerhans of the pancreas: what messages do acetylcholine, glutamate, and GABA transmit? Endocrine 8:213–223
Shi Y, Kanaani J, Menard-Rose V et al (2000) Increased expression of GAD65 and GABA in pancreatic beta-cells impairs first-phase insulin secretion. Am J Physiol Endocrinol Metab 279:E684–E694
Smismans A, Schuit F, Pipeleers D (1997) Nutrient regulation of gamma-aminobutyric acid release from islet beta cells. Diabetologia 40:1411–1415
Winnock F, Ling Z, De Proft R et al (2002) Correlation between GABA release from rat islet beta-cells and their metabolic state. Am J Physiol Endocrinol Metab 282:E937–E942
Kawai, Unger RH (1983) Effects of gamma-aminobutyric acid on insulin, glucagon, and somatostatin release from isolated perfused dog pancreas. Endocrinology 113:111–113
Gilon P, Bertrand G, Loubatieres-Mariani MM, Remacle C, Henquin JC (1991) The influence of gamma-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology 129:2521–2529
Robbins MS, Grouse LH, Sorenson RL, Elde RP (1981) Effect of muscimol on glucose-stimulated somatostatin and insulin release from the isolated, perfused rat pancreas. Diabetes 30:168–171
Adeghate E, Ponery AS (2002) GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell 34:1–6
Gomez R, Asnis N, Tannhauser SL, Barros HM (1999) GABA agonists differentially modify blood glucose levels of diabetic rats. Jpn J Pharmacol 80:327–331
Maechler P, Wollheim CB (2001) Mitochondrial function in normal and diabetic beta-cells. Nature 414:807–812
Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P (2004) Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 145:667–678
Wang Q, Li L, Xu E, Wong V, Rhodes C, Brubaker PL (2004) Glucagon-like peptide-1 regulates proliferation and apoptosis via activation of protein kinase B in pancreatic INS-1 beta cells. Diabetologia 47:478–487
Ashcroft FM, Ashcroft SJ, Harrison DE (1988) Properties of single potassium channels modulated by glucose in rat pancreatic beta-cells. J Physiol 400:501–527
Theler JM, Mollard P, Guerineau N et al (1992) Video imaging of cytosolic Ca2+ in pancreatic beta-cells stimulated by glucose, carbachol, and ATP. J Biol Chem 267:18110–18117
Ashcroft FM, Rorsman P (1989) Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol 54:87–143
Reetz A, Solimena M, Matteoli M, Folli F, Takei K, De Camilli P (1991) GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J 10:1275–1284
Hayashi M, Yamada H, Uehara S et al (2003) Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem 278:1966–1974
Uehara S, Muroyama A, Echigo N et al (2004) Metabotropic glutamate receptor type 4 is involved in autoinhibitory cascade for glucagon secretion by alpha-cells of islet of Langerhans. Diabetes 53:998–1006
Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH (1984) Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 74:2296–2299
Kaneko K, Shirotani T, Araki E et al (1999) Insulin inhibits glucagon secretion by the activation of PI3-kinase in In-R1-G9 cells. Diabetes Res Clin Pract 44:83–92
Kisanuki K, Kishikawa H, Araki E et al (1995) Expression of insulin receptor on clonal pancreatic alpha cells and its possible role for insulin-stimulated negative regulation of glucagon secretion. Diabetologia 38:422–429
Greenbaum CJ, Havel PJ, Taborsky GJ Jr, Klaff LJ (1991) Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J Clin Invest 88:767–773
Bonner-Weir S, Orci L (1982) New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31:883–889
Raskin P, Unger RH (1978) Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med 299:433–436
Johnson DG, Goebel CU, Hruby VJ, Bregman MD, Trivedi D (1982) Hyperglycemia of diabetic rats decreased by a glucagon receptor antagonist. Science 215:1115–1116
Acknowledgements
This study was supported by research operating grants from the Canadian Institute for Health research (CIHR) to W.-Y. Lu, and to Q. Wang, respectively. H. Dong was supported by the Sunnybrook Trust Fellowship. M. Kumar is the recipient of the Canadian Diabetes Association Fellowship. W.-Y. Lu is a New Investigator of CIHR. Q. Wang is a recipient of a Canadian Diabetes Association Scholarship and is supported by New Investigator and Reuben & Helene Dennis Scholar programs of the Banting and Best Diabetes Research Centre. The authors thank J. Ng for technical assistance. The authors are grateful to R. G. Tsushima, P. Brubaker (Department of Physiology, Medicine, University of Toronto) and P. Doherty (St Michael’s Hospital, Toronto, Canada) for critical reading of the manuscript. H. Dong and M. Kumar contributed equally to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, H., Kumar, M., Zhang, Y. et al. Gamma-aminobutyric acid up- and downregulates insulin secretion from beta cells in concert with changes in glucose concentration. Diabetologia 49, 697–705 (2006). https://doi.org/10.1007/s00125-005-0123-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-0123-1