Abstract
Aims/hypothesis
Recent studies suggest that donor endothelial cells may contribute to islet graft revascularisation. Since islet endothelial cells disappear during culture, we hypothesised that transplantation of islets without prior culture is beneficial for their engraftment.
Methods
Cultured (4–7 days) or freshly isolated islets (<4 h after donor pancreas extirpation) were syngeneically transplanted into Wistar–Furth rats and C57Bl/6 mice beneath the renal capsule. Islet graft revascularisation was evaluated by measuring vascular density, blood flow and tissue oxygen tension. Islet graft function was investigated by a minimal islet mass model in inbred mice (C57Bl/6).
Results
Four days after implantation, the partial pressure of oxygen (pO2) in the transplanted cultured islets was less than 10 mmHg (1.33 kPa), but tended to be higher in grafts composed of freshly isolated islets. The pO2 in the grafts of freshly isolated islets had more than doubled 4 weeks later, whereas the pO2 in the grafts of cultured islets remained at values similar to those recorded 4 days after transplantation. Transplanted freshly isolated islets also had a higher vascular density than transplanted cultured islets (∼40 vs ∼25% of that in endogenous islets) when investigated 1 month post-implantation. When applying a minimal islet mass model in inbred mice, 200 freshly isolated islets cured alloxan-diabetic mice in all cases, whereas only 33% of the group receiving similar numbers of cultured islets were cured.
Conclusions/interpretation
Transplantation of pancreatic islets without prior culture is beneficial for their vascular engraftment and function.
Similar content being viewed by others
Introduction
For many years, the clinical outcome of allogeneic islet transplantations was very poor, with less than 10% of the recipients being insulin-independent after 1 year [1]. However, the recently introduced Edmonton Protocol, which applies a steroid- and cyclosporine-free immunosuppressive regime, has improved insulin independence 1 year post-transplantation to approximately 80% [1–3]. The Edmonton Protocol used freshly isolated islets for transplantation [2]. In most former, less successful islet transplant protocols, cultured islets have been used for transplantation, since in vitro culture is often practical from a logistic point of view [4] and may reduce islet immunogenicity by depletion of viable haematogenous and lymphoid cells [5]. Concerns have also been raised regarding the common pronounced exocrine contamination of freshly isolated islet transplants [6].
Endogenous islets normally have a complex architecture of capillaries, which ensures that no portion of the islet is more than one cell away from arterial blood [7]. This unique capillary network is crucial for the supply of oxygen and nutrients to the islet cells and for the dispersal of islet hormones to the systemic circulation [8]. The islet capillary system is, however, disrupted by isolation, and during in vitro culture islet endothelial cells de-differentiate or die [9–11]. The revascularisation process of islets is generally thought to occur over a period of 7–14 days post-transplantation [12–14]. The newly formed blood vessels were earlier considered to originate from recipient blood vessels [15]. However, recent studies suggest that endothelial cells originating from the donor may also contribute significantly, and be important for the revascularisation process [16, 17]. In our previous studies, we have observed that grafts composed of cultured rodent or human islets are not sufficiently revascularised, which results in a low graft oxygen tension and tissue acidosis [18–22]. The present study tested the hypothesis that syngeneic islet grafts composed of freshly isolated rodent islets become more efficiently revascularised than islets transplanted after culture, and that this might result in an improved islet graft function.
Materials and methods
Animals
Male inbred Wistar–Furth rats weighing 300–350 g, and male inbred C57Bl/6 mice weighing 25–30 g were purchased from B&K, Sollentuna, Sweden. The rats and mice had free access to water and pelleted food, and were housed in a room with a 12-h light/dark cycle and 70% humidity throughout the course of the study. All experiments were approved by the local animal ethics committee of Uppsala University, Uppsala, Sweden.
Islet isolation and transplantation
Pancreatic islets were isolated by collagenase (Boehringer-Mannheim; Mannheim, Germany) digestion, as previously described [23]. Some of the islets were cultured free-floating in groups of ∼150 islets in RPMI 1640 medium (Sigma-Aldrich, Irvine, UK) supplemented with 10% (vol/vol) fetal calf serum (Sigma-Aldrich) prior to transplantation. The medium was changed every second day. At the time of transplantation, 250 (rat) or 200 (mouse) freshly isolated (<4 h after pancreas extirpation) or cultured (4–7 days of culture) islets were packed in a braking pipette and implanted beneath the renal capsule on the dorsal side of the left kidney of syngeneic normoglycaemic rats that had been anaesthetised with pentobarbital (60 mg/kg, i.p., Apoteket, Umeå, Sweden) or of syngeneic alloxan-diabetic mice that had been anaesthetised with Avertin (0.02 ml/g i.p, of a 2.5% [vol/vol] solution of 10 g 97% [vol/vol] 2,2,2,-tribromoethanol [Sigma-Aldrich] in 10 ml of 2-methyl-2-butanol [Kemila, Stockholm, Sweden]). For practical reasons, normoglycaemic rats were, used as recipients in the engraftment studies, since there is no difference in revascularisation and oxygenation of transplanted islets, compared to when the islets are implanted to cure diabetic recipients instead [19, 24]. In all cases exocrine contamination of the transplanted islets was avoided as much as possible, since this has previously been observed to have a negative influence on the engraftment process of transplanted islets [25].
Analysis of blood glucose concentration
Glucose reagent strips were used to measure blood glucose concentrations in venous blood obtained from the cut tip of the tail of the animals (MediSense; Baxter Travenol, Deerfield, IL, USA). Blood glucose values above the detection range were set to 27.4 mmol/l, which according to the technical data supplied by the manufacturer is the highest reliable blood glucose concentration evaluable by this device.
Oxygen tension in endogenous islets
Non-transplanted rats were anaesthetised by thiobutabarbital (Inactin, 120 mg/kg i.p.; Research Biochemicals International, Natick, MA, USA), placed on an operating table maintained at body temperature (37°C), and tracheotomised. Polyethylene catheters were inserted into the left femoral artery and the left femoral vein. The arterial catheter was connected to a Statham P23dB pressure transducer to continuously monitor the mean arterial blood pressure throughout the experiment. The femoral vein catheter was used to infuse saline (5 ml kg−1 h−1) to substitute for body fluid loss. The abdomen was opened by a mid-line incision, and the pancreas was immobilised over a hollow cylindrical plastic block attached to the operating table. The pancreas was then continuously superfused with mineral oil (Apoteket) at body temperature to prevent desiccation of the tissue. After allowing the mean arterial pressure to stabilise, 0.8-ml sterile-filtered 2% (wt/vol) neutral red (Kebo Grave, Stockholm, Sweden) was injected intravenously to selectively stain the islets within the pancreas. This dye has previously been evaluated and has been shown not to affect pancreatic oxygenation, whole pancreatic and islet blood flow, or glucose homeostasis [26]. The rats were then allowed to rest for 30 min to minimise the influence of surgical stress and neutral red administration on the measurement of partial pressure of oxygen (pO2).
The pO2 was measured by modified Clark microelectrodes (outer tip diameter 2–6 μm; Unisense, Aarhus, Denmark) [18, 27]. The microelectrodes were calibrated in water saturated with Na2S2O5 or air at 37°C before and after the experiment. The drift of the microelectrode recordings was <0.5%/h. The microelectrode tip was inserted into the islets and the exocrine pancreas with a micromanipulator under a stereo microscope. Measurements of pO2 were performed in three to six islets in each animal. Multiple measurements (≥3) were frequently performed in each islet, and the mean of these measurements was then calculated to obtain the pO2 in one islet. The mean of the pO2 values in one animal was treated as one experiment in the subsequent statistical analysis.
Oxygen tension and blood flow in islet grafts
Four days or 1 month post-transplantation, the transplanted rats were anaesthetised by thiobutabarbital (Inactin, 120 mg/kg i.p.; Research Biochemicals International) and surgically prepared similarly to the non-transplanted rats (see above). However, in this case the abdomen was opened by a left subcostal flank incision, and the left kidney, bearing the islet graft, was immobilised in a plastic cup attached to the operating table. The kidney and the islet graft were embedded in cotton and mineral oil (Apoteket) to prevent heat loss and desiccation. The animals were then allowed to rest for 30 min to minimise the influence of surgical stress on the subsequent measurements.
Repeated measurements (n≥10) of pO2 were conducted in the transplanted islets and adjacent renal parenchyma, and the mean was treated as one experiment. In conjunction with the pO2 measurements, the blood flow in the islet graft and the adjacent renal cortex was recorded by laser-Doppler flowmetry (PF 4001-2, Perimed, Stockholm, Sweden). The blood flow in the islet graft and the adjacent renal cortex was determined by repeated measurements (n≥3) in each animal, and again the mean was treated as one experiment.
Endothelial staining
Following blood flow and pO2 measurements, the 1-month-old islet grafts were retrieved for histological examination. The pancreas was also retrieved from the age-matched non-transplanted rats. The pancreas and the islet grafts were fixed in 10% (vol/vol) formalin and embedded in paraffin. Sections (5 μm thick) were stained for endothelial cells by the lectin Bandeiraea simplicifolia [28]. In brief, the sections were incubated with normal goat serum (NGS; Dakopatts, Glostrup, Denmark), diluted 1:20 with Tris buffered saline (TBS), and kept for 1 h in a moist chamber at room temperature (20°C). Biotinylated forms of lectin from Bandeiraea simplicifolia (Sigma-Aldrich), diluted 1:100 in TBS, were applied to the sections overnight at 4°C. The sections were then washed and incubated with Vectastain ABC-AP kit (Vector Laboratories, Burlingame, CA, USA) for 30 min in a moist chamber at room temperature. They were then washed again and the chromogen Vector Red (Vector Laboratories) was applied to the sections and left for 30 min to develop in a moist chamber at room temperature. Thereafter, the slides were washed in TBS, counterstained with haematoxylin, dehydrated and mounted with Mountex (Histolab Products, Gothenburg, Sweden).
Evaluation of vascular density
In each rat, more than 15 endogenous islets or more than 2 mm2 of islet grafts were randomly chosen from the histological sections and evaluated. The blood vessels in endogenous and transplanted islets were counted in a light microscope at a magnification of 600× and by an examiner unaware of the origin of the sections. Stroma surrounded the individual islets in the grafts. The microvessels in the transplanted islets and stroma were counted separately. The respective fractions of islets and connective tissue in the islet grafts were determined by a direct point counting technique [29]. For this purpose, the intersections overlapping the stroma and endocrine cells within the islet grafts were counted (magnification 600×). At least ten fields (corresponding to ∼1,200 intersections) were counted in each islet graft. The areas of the investigated endogenous islets and grafted islets were determined using a computerised system for morphometry (MOP-Videoplan; Carl Zeiss, Stockholm, Sweden). Vascular density, i.e. the number of blood vessels per measured islet or graft area (mm2), was then calculated.
Evaluation of graft volume
Renal subcapsular islet grafts, composed of 250 freshly isolated or 250 cultured rat islets, were retrieved 1 month post-transplantation and prepared for histological evaluation. The islet grafts were formalin-fixed, paraffin-embedded, consecutively sectioned (5 μm) and stained with haematoxylin and eosin. The total graft volumes and the fractions constituting endocrine cells were estimated using a computerised system for morphometry (MOP-Videoplan; Carl Zeiss, Stockholm, Sweden), as previously described [30].
Insulin, vascular endothelial growth factor and basic fibroblast growth factor content in freshly isolated and cultured rat islets
Groups of 125 freshly isolated or cultured rat islets (5 days of culture) were placed in 500-μl Hanks and sonicated. Homogenates were then stored at −70°C until analysis. The insulin content of the homogenates was measured with a rat insulin ELISA (Mercodia, Uppsala, Sweden), whereas the vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) contents were analysed with a mouse VEGF ELISA and human bFGF ELISA respectively (R&D Systems, Minneapolis, MN, USA). Amino acid homology greater than 90% between species is recommended by the manufacturer for their ELISAs to ascertain specific cross-reactivity. The amino acid homology for rat and mouse VEGF is 98%, and for rat and human bFGF 95.5%.
Insulin, vascular endothelial growth factor and basic fibroblast growth factor content in rat islet grafts
Four-day-old or 1-month-old islet grafts were dissected free from the surrounding renal parenchyma, placed in 1 ml acid ethanol (0.18 mol/l HCl in 70% vol/vol ethanol) and sonicated to disrupt the islet cells. The samples were left to extract overnight at 4°C, and then stored in a freezer until analysed by ELISA (see above).
Minimal islet mass
Mice were given an intravenous injection of alloxan (75 mg/kg; Sigma-Aldrich) 5 days prior to transplantation and were considered diabetic if they had non-fasting blood glucose concentrations above 16.7 mmol/l at this time. The number of transplanted islets (200) was chosen on the basis of our previous studies in this strain, e.g. [24], and intended to reach an islet mass insufficient for full reversal of hyperglycaemia in most of the diabetic recipients receiving cultured islets. The body weights and blood glucose concentrations of the transplanted animals were measured every fifth day up to 1 month post-transplantation. Animals cured from diabetes were defined as those with non-fasting blood glucose concentrations lower than 11.1 mmol/l. The graft-bearing kidneys were removed on all the cured animals 1 month post-transplantation to ascertain that they would subsequently return to hyperglycaemia (>16.7 mmol/l) and that the improved blood glucose concentrations were not merely the result of regained function in the endogenous pancreas.
Statistical analysis
Values are expressed as the mean±SEM. Multiple comparisons between data were performed using ANOVA and Fisher’s protected least significant difference test (SigmaStat 2.0, SPSS, Chicago, IL, USA). When only two groups were compared, probabilities (p) of chance differences between the experimental groups were calculated using Student’s unpaired two-tailed t-test. For all comparisons, p values of less than 0.05 were considered statistically significant.
Results
Animal characteristics
All rats allocated to the islet graft blood flow and pO2 measurements weighed ∼300 g and were normoglycaemic with blood glucose concentrations of ∼6.0 mmol/l. The mean arterial blood pressure was similar in all groups of rats and ranged between 100 and 130 mmHg.
Oxygen tension in endogenous and transplanted rat islets
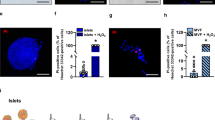
The pO2 in endogenous islets was ∼45 mmHg (6.0 kPa) (Fig. 1), whereas the pO2 in the surrounding exocrine parenchyma was 28.8±2.0 mmHg (3.83±0.27 kPa; n=7). All transplanted islets, irrespective of whether investigated 4 days or 1 month post-transplantation, had a markedly lower pO2 (Fig. 1). However, already 4 days after transplantation, islet grafts composed of freshly isolated islets tended (p=0.09) to have higher pO2 than corresponding grafts composed of cultured islets. One month post-transplantation, the pO2 in the islet grafts composed of freshly isolated islets had more than doubled, whereas the pO2 in grafts composed of cultured islets remained at values similar to those recorded 4 days after transplantation (Fig. 1). The pO2 in the renal cortex adjacent to the islet grafts was similar in all animals [19.1±0.9 mmHg (2.54±0.12 kPa), n=30].
Oxygen tension in endogenous rat islets (closed bar) and in 4-day-old and 1-month-old syngeneic islet grafts composed of cultured (hatched bar) or freshly isolated (open bar) rat islets. All values are expressed as means±SEM for seven to eight experiments in each group. *p<0.05 when compared to native islets; # p<0.05 when compared to corresponding 4-day-old grafts; † p<0.05 when compared to corresponding 1-month-old grafts of cultured islets
Blood flow in rat islet grafts
Islet graft blood flow (Fig. 2) increased from ∼35 to ∼60% of that in the renal cortex between 4 days and 1 month post-transplantation. The blood perfusion of grafts composed of freshly isolated and cultured islets did not differ at either point of time. Renal cortical blood flow was similar in all groups.
Blood flow in 4-day-old and 1-month-old syngeneic grafts composed of cultured (closed bar) or freshly isolated rat islets (hatched bar), and in the adjacent renal cortex. All values are expressed as means±SEM for seven to eight experiments in each group. *p<0.05 when compared to corresponding 4-day-old islet grafts
Vascular density and volume of rat islet grafts
Figures 3 and 4 show that islet grafts composed of cultured islets had a vascular density only 25% of that in endogenous islets when investigated 1 month post-transplantation, whereas transplanted freshly isolated islets had a vascular density at the same time point after implantation of about 40% of that in endogenous islets. The vascular density in the stroma surrounding the individual islets in the grafts was markedly higher than in the endocrine parts per se, and it was similar in grafts composed of cultured and non-cultured islets. The percentage of stroma did not differ between islet grafts composed of cultured or non-cultured islets (25±3 vs 22±3%; n=7 in both groups). Neither did the endocrine volume of the 1-month-old grafts composed of cultured and freshly isolated islets differ (375±28 nl, n=8 and 368±16 nl, n=7, respectively).
Vascular density in endogenous rat islets and endocrine parts of 1-month-old syngeneic islet grafts composed of cultured or freshly isolated rat islets (closed bars), and in the stroma of the same islet grafts (hatched bars). All values are expressed as means±SEM for seven to eight experiments in each group. *p<0.05 when compared to native islets; # p<0.05 when compared to grafts composed of cultured islets
Insulin, vascular endothelial growth factor and basic fibroblast growth factor content in isolated rat islets and rat islet grafts
The insulin content in isolated islets decreased during 5 days of culture (Fig. 5a). Four days after transplantation, the insulin content in the islets was even lower than during in vitro culture, and was similar in grafts composed of non-cultured and cultured islets. The insulin content of grafts composed of freshly isolated islets, but not of those composed of cultured islets, decreased further between 4 days and 1 month post-transplantation. However, the insulin content did not differ statistically between the 1-month-old grafts composed of freshly isolated and cultured islets.
Insulin (a), vascular endothelial growth factor (VEGF) (b) and basic fibroblast growth factor (bFGF) (c) content of cultured (closed boxes) and freshly isolated (open boxes) rat islets in vitro, 4 days or 1 month post-transplantation. All values are expressed as means±SEM for seven to eight experiments in each group. *p<0.05 when compared to corresponding cultured islets; # p<0.05 when compared to corresponding islets in vitro; † p<0.05 when compared to corresponding islets 4 days post-transplantation
The VEGF content of isolated islets increased sixfold during 5 days of culture (Fig. 5b). Four days after transplantation, the VEGF content was ∼0.15 pg/islet in grafts composed of freshly isolated islets and in grafts composed of cultured islets. One month post-transplantation, VEGF in the transplanted islets was no longer detectable (values <0.02 pg/islet for all grafts).
Islet bFGF content was not effected by culture, but decreased soon after transplantation (day 4) in islets transplanted as freshly isolated specimens and in islets transplanted after a culture period (Fig. 5c). In 1-month-old grafts, the bFGF content was again higher, and there was a tendency (p=0.07) for islet grafts composed of freshly isolated islets to have a higher bFGF content than grafts of cultured islets.
Minimal islet mass in mice
Treatment with alloxan rapidly increased blood glucose concentrations in treated mice to above 20 mmol/l (Table 1). At the time of transplantation, all mice had similar blood glucose concentrations, but those randomly assigned to receive cultured islets weighed slightly less than those receiving freshly isolated islets (Table 1). Transplantation of 200 freshly isolated mice islets fully reversed the hyperglycaemia in all (n=8) alloxan-diabetic recipients within 1 month of transplantation (Fig. 6). In contrast, only one-third (n=6) of the alloxan-diabetic mice that received 200 cultured islets was cured. One month post-transplantation, animals receiving cultured islets had higher mean blood glucose concentrations than those receiving freshly isolated islets (Table 1). Due to overt hyperglycaemia, two animals receiving cultured islets died during the course of the study and had to be excluded, whereas all mice receiving freshly isolated islets survived the study period. Removal of the kidney bearing the graft 1 month post-transplantation, reversed all cured mice to diabetes (>16.7 mmol/l).
Cumulative incidence of reversal to normoglycaemia in alloxan-diabetic mice receiving 200 cultured (closed symbols) or freshly isolated islets (open symbols). Blood glucose concentrations were monitored every fifth day during a month-long period after transplantation. The animals were considered to be cured when blood glucose concentrations were <11.1 mmol/l
Discussion
In this study, using a syngeneic rat model, we observed a higher vascular density and oxygen tension in 1-month-old islet grafts composed of freshly isolated islets than in grafts composed of cultured islets. This suggests that revascularisation is better in transplanted freshly isolated islets, resulting in a more adequate supply of oxygen to the transplanted islet cells. When applying a minimal islet mass model in inbred mice, we also observed that grafts composed of freshly isolated islets had a higher capacity to cure alloxan-induced diabetes than corresponding numbers of cultured islets. Despite better vascular engraftment, the volume and insulin content did not differ between rat islet grafts composed of freshly isolated or cultured islets. However, the higher vascular density in the 1-month-old grafts of freshly isolated islets is likely to improve the delivery of insulin to the blood stream.
There was no difference in the blood perfusion of rat islet grafts composed of freshly isolated and cultured islets either at 4 days or 1 month post-transplantation. It should, however, be noted that the blood flow measured by laser-Doppler flowmetry represents not only the nutritive blood flow to the endocrine cells, but total blood perfusion, i.e. all moving blood cells within the illuminated tissue. A large number of the intragraft capillaries was stroma capillaries, see [20], which are likely to contribute substantially to total graft blood perfusion. In contrast, it could be expected that the blood flow in capillaries in the endocrine parts mainly contributes to the delivery of oxygen to the endocrine cells, due to limitations of oxygen diffusion distance [31]. Indeed, 1-month-old grafts composed of freshly isolated islets had a higher islet vascular density than corresponding grafts of cultured rat islets, whereas the vascular density in the stroma was similar in grafts composed of freshly isolated and cultured rat islets. Moreover, the oxygen tension measurements suggested that the higher number of blood vessels in the endocrine parts of 1-month-old grafts of freshly isolated rat islets were not merely explained by remnant donor endothelial cells, but also by functional blood-perfused capillaries. In 4-day-old rat islet grafts, only blood flow and oxygen tension were recorded, since we deemed it likely that remnant donor endothelial cells, especially in transplanted freshly isolated islets, would confound measurements of vascular density. In contrast to our results, a previous histological study in rats [13] suggested that transplanted cultured islets may become revascularised more slowly than freshly isolated islets, but that all islets become fully revascularised within 1 week. However, the authors [13] did not mention whether endocrine and connective tissue parts were evaluated separately.
In an attempt to explain why freshly isolated islets become more efficiently revascularised than cultured islets following transplantation, we measured concentrations of angiogenic factors in freshly isolated and cultured rat islets, as well as in 4-day-old and 1-month-old rat islet grafts. The process of angiogenesis has been extensively studied in different experimental setups, and the most important growth factors involved seem to be VEGF and bFGF [32–34]. Consistent with previous studies [35, 36], we found that the islet production of VEGF increased markedly during culture. Transplanted islets also seem to have an increased expression of VEGF in the immediate post-transplantation period [37], which is consistent with the fact that the VEGF concentration is elevated during the early angiogenic phase. However, in the present study the VEGF content was similar in islet grafts composed of freshly isolated or cultured rat islets when investigated during active angiogenesis 4 days after transplantation. Thus, differences in VEGF production seem unlikely to explain the better revascularisation of islets transplanted immediately after isolation. This is also indirectly supported by results from a previous study, where in vivo blockage of actions of endogenous VEGF did not impair the revascularisation of freely transplanted islets [38]. Notably, however, the induction of marked VEGF hyperexpression in islets prior to transplantation through gene transfer seems to improve both islet revascularisation and function [39].
Freshly isolated islets have previously been reported to produce bFGF in vitro [40]. In the present study we observed that the bFGF production of isolated rat islets is not affected by subsequent culture. A rapid decline in islet graft bFGF expression has been reported to occur 3–5 days post-transplantation in grafts composed of freshly isolated islets [40]. In our study, the production of bFGF in 4-day-old rat islet grafts was similar in grafts composed of freshly isolated and cultured islets. One-month-old rat islet grafts had a higher production of bFGF than 4-day-old islet grafts. There was also a tendency (p=0.07) for 1-month-old grafts composed of freshly isolated rat islets to have a higher bFGF content than those of cultured islets. This may have contributed to the better revascularisation of grafts composed of freshly isolated islets, since bFGF has been reported to improve blood vessel stability [41].
The formation of a highly vascularised stroma in the grafts is probably due to growth factors excreted by the transplanted islets, whereas the reason(s) for the defective revascularisation of the endocrine tissue per se remain(s) largely unclear. However, some clues were provided in the present study, where transplanted freshly isolated islets were more efficiently revascularised than transplanted cultured islets. This suggests that remnant donor endothelial cells are indeed important in the revascularisation process. It could be argued that the remaining microvessels in freshly isolated islets serve as channels for the migration of new vessels, which may induce the process of revascularisation. Remaining endothelial cells may also attract and become incorporated within the newly formed microvessels [16, 17]. Furthermore, it should be noted that macrophages residing within the pancreatic islets are known to disappear during culture [9]. Monocytes and tissular macrophages seem strongly involved in adult angiogenesis due to their local secretion of metalloelastases, which cause the formation of capillary lumens through local tunnelling in the parenchyma [42, 43]. Monocytes and macrophages also contribute to the local pool of endothelial progenitor cells [44]. The role of these cells in adult angiogenesis, including that in the revascularisation of pancreatic islets, has recently been demonstrated [45, 46]. Moreover, the macrophage-derived factor matrix metalloproteinase-9 has been reported to be pivotal for the angiogenic switch to occur in insulin-producing tumours [47].
In this study, we compared the revascularisation of syngeneic rat islet grafts composed of freshly isolated islets with that of grafts composed of islets cultured for 4–7 days. Since human islets in most cases nowadays are cultured for a shorter period of time prior to transplantation, it cannot be excluded that such islets still possess some of the angiogenic properties of freshly isolated islets. As indicated in early clinical studies, a culture period, at least overnight, may be valuable in the clinical situation to reduce exocrine contamination [48].
In conclusion, the present results imply that immediate transplantation of islets, without preceding culture, may be advantageous in islet transplantation by improving their vascular engraftment and function. These findings are based on syngeneic transplantation models, where revascularisation can be studied in a standardised manner without interference by factors such as immunosuppression and immunological rejection. The clinical importance of the present results needs to be further evaluated in the human allogeneic setting.
Abbreviations
- bFGF:
-
basic fibroblast growth factor
- pO2 :
-
partial pressure of oxygen
- TBS:
-
Tris buffered saline
- TPU:
-
tissue perfusion units
- VEGF:
-
vascular endothelial growth factor
References
Brendel MD, Hering BJ, Schultz AO, Bretzel RG (2001) International islet transplant registry. Newsletter No. 9, vol. 8. Third Medical Department, Center of Internal Medicine, University Hospital Giessen, Germany, pp 1–19
Shapiro AMJ, Lakey JR, Ryan EA et al (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid immunosuppressive regimen. N Engl J Med 343:230–238
Ryan EA, Lakey JR, Rajotte RV et al (2001) Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 50:710–719
Weir GC, Bonner-Weir S (1997) Scientific and political impediments to successful islet transplantation. Diabetes 46:1247–1256
Kedinger M, Haffen K, Grenier J, Eloy R (1977) In vitro culture reduces immunogenicity of pancreatic endocrine islets. Nature 270:736–738
Gray DW (1989) The role of exocrine tissue in pancreatic islet transplantation. Transpl Int 2:41–45
Bonner-Weir S (1988) Morphological evidence for pancreatic polarity of β-cells within islets of Langerhans. Diabetes 37:616–621
Jansson L (1994) The regulation of pancreatic islet blood flow. Diabetes Metab Rev 10:407–416
Parr E, Bower K, Lafferty K (1980) Changes in cultured mouse thyroid glands and islets of Langerhans. Transplantation 30:135–141
Lukinius A, Jansson L, Korsgren O (1995) Ultrastructural evidence for blood microvessels devoid of an endothelial cell lining in transplanted pancreatic islets. Am J Pathol 146:429–435
Furuya H, Kimura T, Murakami M, Katayama K, Hirose K, Yamaguchi A (2003) Revascularization and function of pancreatic islet isografts in diabetic rats following transplantation. Cell Transplant 12:537–544
Menger MD, Jäger S, Walter P, Feifel G, Hammersen F, Messmer K (1989) Angiogenesis and hemodynamics of microvasculature of transplanted islets of Langerhans. Diabetes 38(Suppl 1):199–201
Mendola JF, Goity C, Fernandez-Alvarez J et al (1994) Immunocytochemical study of pancreatic islet revascularization in islet isograft: effect of hyperglycemia of the recipient and of in vitro culture of islets. Transplantation 57:725–730
Merchant FA, Diller KR, Aggarwal SJ, Bovik AC (1997) Angiogenesis in cultured and cryopreserved pancreatic islet grafts. Transplantation 63:1652–1660
Vajkoczy P, Olofsson AM, Lehr H-A et al (1995) Histogenesis and ultrastructure of pancreatic islet graft microvasculature: evidence for graft revascularization of host origin. Am J Pathol 146:1397–1405
Linn T, Schneider K, Hammes HP et al (2003) Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB J 17:881–883
Brissova M, Fowler M, Wiebe P et al (2004) Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes 53:1318–1325
Carlsson P-O, Liss P, Andersson A, Jansson L (1998) Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes 47:1027–1032
Carlsson P-O, Palm F, Andersson A, Liss P (2001) Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of implantation site. Diabetes 50:489–495
Mattsson G, Jansson L, Carlsson P-O (2002) Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes 51:1362–1366
Carlsson P-O, Nordin A, Palm F (2003) pH is decreased in transplanted rat pancreatic islets. Am J Physiol Endocrinol Metab 284:E499–E504
Carlsson P-O, Palm F, Mattsson G (2002) Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab 87:5418–5423
Andersson A (1978) Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia 14:397–404
Mattsson G, Jansson L, Nordin A, Carlsson P-O (2003) Impaired revascularization of transplanted mouse pancreatic islets is chronic and glucose-independent. Transplantation 75:736–739
Heuser M, Wolf B, Vollmar B, Menger MD (2000) Exocrine contamination of isolated islets of Langerhans deteriorates the process of revascularization after free transplantation. Transplantation 69:759–761
Carlsson P-O, Jansson L, Östenson C-G, Källskog Ö (1997) Islet capillary blood pressure increase mediated by hyperglycemia in NIDDM GK rats. Diabetes 46:947–952
Revsbech NP (1989) An oxygen microsensor with a guard cathode. Limnol Oceanogr 34:474–478
Mattsson G, Carlsson P-O, Olausson K, Jansson L (2002) Histological markers for endothelial cells in endogenous and transplanted rodent pancreatic islets. Pancreatology 2:155–162
Weibel ER (1979) Practical methods for biological morphometry. In: Weibel ER (ed) Stereological methods. Academic, London, pp 101–161
Sandler S, Jansson L (1987) Blood flow measurements in autotransplanted pancreatic islets of the rat: impairment of the blood perfusion of the graft during hyperglycemia. J Clin Invest 80:17–21
Dionne KE, Colton CK, Yarmush ML (1989) Effect of oxygen on isolated pancreatic tissue. ASAIO Trans 35:739–741
Bruick RK, McKnight SL (2001) Building better vasculature. Genes Dev 15:2497–2502
Ferrara N (2001) Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol 280:C1358–C1366
Poole TJ, Finkelstein EB, Cox CM (2001) The role of FGF and VEGF in angioblast induction and migration during vascular development. Dev Dyn 220:1–17
Gorden DL, Mandriota SJ, Montesano R, Orci L, Pepper MS (1997) Vascular endothelial growth factor is increased in devascularized rat islets of Langerhans in vitro. Transplantation 63:436–443
Vasir B, Aiello LP, Yoon KH, Quickel RR, Bonner-Weir S, Weir GC (1998) Hypoxia induces vascular endothelial growth factor gene and protein expression in cultured rat islet cells. Diabetes 47:1894–1903
Vasir B, Jonas JC, Steil GM et al (2001) Gene expression of VEGF and its receptors Flk-1/KDR and Flt-1 in cultured and transplanted rat islets. Transplantation 71:924–935
Schramm R, Yamauchi J, Vollmar B, Vajkoczy P, Menger MD (2003) Blockade of in vivo VEGF-KDR/flk-1 signaling does not affect revascularization of freely transplanted pancreatic islets. Transplantation 75:239–242
Zhang N, Richter A, Suriawinata J et al (2004) Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes 53:963–970
Vasir B, Reitz P, Xu G, Sharma A, Bonner-Weir S, Weir GC (2000) Effects of diabetes and hypoxia on gene markers of angiogenesis (HGF, cMET, uPA and uPAR, TGF-alpha, TGF-beta, bFGF and Vimentin) in cultured and transplanted rat islets. Diabetologia 43:763–772
Compagni A, Wilgenbus P, Impagnatiello MA, Cotten M, Christofori G (2000) Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res 60:7163–7169
Moldovan NI (2002) Role of monocytes and macrophages in adult angiogenesis: a light at the tunnel’s end. J Hematother Stem Cell Res 11:179–194
Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE (2000) Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res 87:378–384
Rehman J, Li J, Orschell CM, March KL (2003) Peripheral blood ‛endothelial progenitor cells’ are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107:1164–1169
Murayama T, Asahara T (2002) Bone marrow-derived endothelial progenitor cells for vascular regeneration. Curr Opin Mol Ther 4:395–402
Contreras JL, Smyth CA, Eckstein C et al (2003) Peripheral mobilization of recipient bone marrow-derived endothelial progenitor cells enhances pancreatic islet revascularization and engraftment after intraportal transplantation. Surgery 134:390–398
Bergers G, Brekken R, McMahon G et al (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2:737–744
Matas AJ, Sutherland DE, Steffes MW, Najarian JS (1976) Short-term culture of adult pancreatic fragments for purification and transplantation of islets of Langerhans. Surgery 80:183–191
Acknowledgements
This work was supported by grants from The Swedish Research Council (72XD-15043), The Juvenile Diabetes Research Foundation, The Swedish Diabetes Association, The Swedish Society of Medicine, The Novo Nordic Fund, The Swedish Juvenile Diabetes Fund, The Anér Foundation, The Clas Groschinsky Memorial fund, The Åke Wiberg Foundation, The Magnus Bergvall Foundation, The Siblings Svensson Foundation and The Family Ernfors Fund. The skilled technical assistance of Astrid Nordin and Eva Törnelius is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olsson, R., Carlsson, PO. Better vascular engraftment and function in pancreatic islets transplanted without prior culture. Diabetologia 48, 469–476 (2005). https://doi.org/10.1007/s00125-004-1650-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1650-x