Abstract
Aims/hypothesis
Accumulating evidence indicates that replacement of C-peptide in type 1 diabetes ameliorates nerve and kidney dysfunction, but the molecular mechanisms involved are incompletely understood. C-peptide shows specific binding to a G-protein-coupled membrane binding site, resulting in Ca2+ influx, activation of mitogen-activated protein kinase signalling pathways, and stimulation of Na+, K+-ATPase and endothelial nitric oxide synthase. This study examines the intracellular signalling pathways activated by C-peptide in human renal tubular cells.
Methods
Human renal tubular cells were cultured from the outer cortex of renal tissue obtained from patients undergoing elective nephrectomy. Extracellular-signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK) and Akt/protein kinase B (PKB) activation was determined using phospho-specific antibodies. Protein kinase C (PKC) and RhoA activation was determined by measuring their translocation to the cell membrane fraction using isoform-specific antibodies.
Results
Human C-peptide increases phosphorylation of ERK1/2 and Akt/PKB in a concentration- and time-dependent manner in renal tubular cells. The C-terminal pentapeptide of C-peptide is equipotent with the full-length C-peptide, whereas scrambled C-peptide has no effect. C-peptide stimulation also results in phosphorylation of JNK, but not of p38 mitogen-activated protein kinase. MEK1/2 inhibitor PD98059 blocks the C-peptide effect on ERK1/2 phosphorylation. C-peptide causes specific translocation of PKC isoforms δ and ɛ to the membrane fraction in tubular cells. All stimulatory effects of C-peptide were abolished by pertussis toxin. The isoform-specific PKC-δ inhibitor rottlerin and the broad-spectrum PKC inhibitor GF109203X both abolish the C-peptide effect on ERK1/2 phosphorylation. C-peptide stimulation also causes translocation of the small GTPase RhoA from the cytosol to the cell membrane. Inhibition of phospholipase C abolished the stimulatory effect of C-peptide on phosphorylation of ERK1/2, JNK and PKC-δ.
Conclusions/interpretation
C-peptide signal transduction in human renal tubular cells involves the activation of phospholipase C and PKC-δ and PKC-ɛ, as well as RhoA, followed by phosphorylation of ERK1/2 and JNK, and a parallel activation of Akt.
Similar content being viewed by others
Introduction
A series of studies during the past decade have presented new aspects of C-peptide physiology. There is evidence to suggest that C-peptide binds to a G-protein-coupled membrane binding site on a number of different cell types [1], thereby triggering Ca2+-dependent intracellular signalling pathways [2] including the mitogen-activated protein (MAP) kinase cascade [3, 4]. This results in subsequent activation of both Na+, K+-ATPase [5–7] and endothelial nitric oxide synthase (eNOS) [5–8]. Activation of these enzyme systems is of particular interest in diabetes, since both are reported to be deficient in this disorder [9–12]. Studies in animal models of diabetes and in patients with type 1 diabetes demonstrate that administration of C-peptide, to yield physiological concentrations, results in a substantial improvement of diabetes-induced functional and structural changes in peripheral nerves [13–15]. In addition, there is evidence to indicate that C-peptide prevents diabetes-induced deficits in nerve fibre regeneration [16], protects against glucose-induced apoptosis of nerve cells and stimulates cellular proliferation [17, 18]. Moreover, C-peptide in replacement doses corrects the characteristic glomerular hyperfiltration seen in the early stages of diabetic nephropathy, reduces urinary excretion of albumin and prevents the development of glomerular hypertrophy in type 1 diabetes [13, 19, 20]. In addition, recent studies have established that C-peptide given in replacement doses to type 1 patients augments skeletal muscle and myocardial blood flow and increases the rate of contraction and the stroke volume of the left ventricle [21–23].
The molecular mechanism by which C-peptide exerts its effects is now becoming clearer. Extracellular signal-regulated kinase (ERK) 1/2 and isoforms of protein kinase C (PKC) are two groups of serine/threonine protein kinases that play important roles in the regulation of cellular functions [24, 25]. C-peptide is reported to stimulate these two pathways. It increases the PKC-dependent phosphorylation of ERK1/2 in Swiss 3T3 fibroblasts [3] and in opossum renal tubular cells [26]. C-peptide also stimulates the phosphorylation of ERK1/2 and p38 MAP kinase in endothelial cells [27], resulting in increased eNOS-mediated synthesis of NO and augmented formation of eNOS protein, secondary to upregulation of eNOS gene transcription, in rat aortic endothelial cells [8]. Moreover, C-peptide stimulates Na+, K+-ATPase activity [6] via PKC-α activation in rat medullary thick ascending limb cells [7]. However, a comprehensive picture of the sequential steps in the C-peptide signalling pathway has not been presented. Moreover, most of the previously reported intracellular effects of C-peptide have been observed in cell models of rodent origin. Consequently, the aim of the present study was to examine, in human renal tubular cells, the sequential steps involved in C-peptide signalling based on the hypothesis that such signalling would involve activation of a G-protein-coupled receptor, phospholipase C, specific PKC isoforms and the MAP kinase system.
Materials and methods
Materials
Human recombinant C-peptide was obtained from Schwarz Pharma (Monheim, Germany). Scrambled C-peptide (the same amino acid residues as in C-peptide, but assembled in random order) and C-terminal pentapeptide (EGSLQ) were from Sigma Genosys (Cambridge, UK). Insulin (Actrapid) was from Novo Nordisk (Denmark). Pertussis toxin (PTX) and verapamil were from Sigma (St Louis, MO, USA). Rottlerin, GF109203X, nifedipine, phorbol myristate acetate (PMA) and PD98059 were from Calbiochem (San Diego, CA, USA). Mouse monoclonal anti-PKC-α, -β, -γ, -δ, -ɛ, -ζ, -η and -θ were from Transduction Laboratories (Lexington, KY, USA). Monoclonal anti-phospho-ERK1/2 (P-Thr202/Tyr204) and polyclonal anti-Akt/protein kinase B (PKB) (P-Sr473) were from New England BioLabs (Beverly, MA, USA). Rabbit polyclonal anti-phospho-JNK (P-Thr183/Tyr185), p38 (P-Tyr188/182) and PKC-δ (Ser643/676) were from Cell Signaling Technology (Beverly, MA, USA). RhoA activation assay kit and mouse monoclonal anti-RhoA antibody were from Upstate Biotechnology (Lake Placid, NY, USA). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse immunoglobulin G was obtained from Bio-Rad Laboratories (Hercules, CA, USA). Reagents for enhanced chemiluminescence were obtained from Amersham (Arlington Heights, IL, USA). All other reagents were of analytical grade (Sigma).
Cell culture
Human renal tubular cells (HRTC) were cultured from the unaffected outer cortex of renal tissue obtained from nondiabetic patients undergoing elective nephrectomy for renal cell carcinoma [28]. The cells were cultured in RPMI 1640 (Life Technologies) supplemented with 10% fetal calf serum, 2 mmol/l L-glutamine, 10 mmol/l HEPES, benzylpenicillin (100 U/ml) and streptomycin (100 μg/ml), and passaged at near confluence by trypsinisation. Growing cells exhibited epithelial morphology with a central nucleus, a granular cytoplasm and cobblestone appearance on light microscopy. Cells from the second and third passages were used for experiments. Tissue collection was undertaken with the informed consent of the subject and approval by the institutional ethics committee.
Cell stimulation
HRTC were serum starved overnight and stimulated with human C-peptide, C-terminal pentapeptide or scrambled human C-peptide. Before stimulation, groups of cells were treated overnight as follows: (1) in the absence or presence of PTX (100 ng/ml); (2) in the absence or presence of 20 μmol/l PD 098059, 20 μmol/l nifedipine or 20 μmol/l verapamil for 20 min in serum-free media; or (3) in the absence or presence of 1 or 10 μmol/l GF109203X, or 20 μmol/l rottlerin for 30 min in serum-free media. Subsequently, cells were washed three times with ice-cold PBS and lysed with ice-cold buffer containing 50 mmol/l HEPES (pH 7.5), 150 mmol/l NaCl, 5 mmol/l EDTA, 10 mmol/l sodium pyrophosphate, 2 mmol/l sodium vanadate, 1% of Triton X-100 and a protease inhibitor cocktail (1 mmol/l phenylmethylsulphonyl fluoride, and 10 μg/ml of each of aprotinin, leupeptin and pepstatin). The lysate was kept on ice for 30 min and centrifuged at 12,000×g for 10 min at 4°C. Protein concentration was determined with a BCA Protein Assay (Pierce, Rockford, IL, USA). Lysates were kept at −80°C before subsequent western blot analysis with appropriate antibodies. For subcellular fractionation experiments, cells were stimulated with 5 nmol/l of human C-peptide, C-terminal pentapeptide of C-peptide or scrambled human C-peptide, 100 nmol/l of insulin, or 100 nmol/l of PMA for 10 min, without or with pretreatment with 1 μmol/l GF109203X or 20 μmol/l rottlerin for 30 min.
Cell fractionation
Nearly confluent HRTC, growing in 100-mm dishes, were serum starved overnight and stimulated with C-peptide in the absence or presence of inhibitors as specified above. After three washings with ice-cold PBS, cells were scraped from dishes into ice-cold buffer containing 20 mmol/l Tris (pH 7.6), 1 mmol/l EDTA and 250 mmol/l sucrose plus a protease inhibitor cocktail. Cells were transferred to 1.5-ml Eppendorf test tubes and homogenised by Pellet Pestle for 1 min (VWR International, Stockholm, Sweden) and by passing through a 21-G syringe needle five times. The cell homogenates were pre-cleared from nuclei and cell debris by centrifugation at 12,000×g for 10 min at 4°C. Supernatants were collected and centrifuged at 150,000×g for 1 h at 4°C, and the supernatants from this step were retained as cytosolic fraction. The pellet was homogenised by Pellet Pestle in the above buffer supplemented with 1% Triton X-100, shake-mixed for 30 min at 4°C, and centrifuged at 180,000×g for 1 h at 4°C. The supernatant containing the membrane-soluble proteins was used for western blot analysis. The remaining pellet contained the Triton-insoluble fraction. All fractions were kept at −80°C before assay.
PKC and RhoA activation assay
PKC and RhoA activation was measured as translocation of PKC isoforms and RhoA from the cytosol to the membrane fraction. Cell membrane Triton-X-100-soluble protein fractions were used to detect PKC and RhoA translocation by western blot with appropriate antibodies. The ability of activated, GTP-bound RhoA to bind with the GST-Rho-binding domain of rhotekin was measured using a commercially available kit (Upstate) according to the manufacturer’s instructions. The GTPγS-loaded sample has been used as a positive control.
Western blot analysis
Aliquots of cell lysate (20 μg of protein) or crude membrane Triton-X-100-soluble fractions (40 μg protein) were resuspended in Laemmli sample buffer. Proteins were then separated by SDS-PAGE, transferred to polyvinylidenedifluoride membranes (Millipore, MA, USA), blocked with 7.5% nonfat milk, washed with TBST (10 mmol/l Tris–HCl, 100 mmol/l NaCl, 0.02% Tween 20) and incubated with appropriate primary antibodies overnight at 4°C. Membranes were washed with TBST and incubated with an appropriate secondary antibody. Proteins were visualised by enhanced chemiluminescence and quantified by densitometry.
Statistical analysis
Data are presented as means ± SE. Student’s t-test was used to assess differences between two treatments within a group. All other differences were evaluated by one-way ANOVA. Fisher’s least significant difference post hoc analysis was used to identify significant differences. A p value of less than 0.05 was considered statistically significant.
Results
Effects of C-peptide on ERK1/2, JNK and p38 MAP kinase phosphorylation
The present study demonstrates that human renal tubular cells in primary culture respond to acute C-peptide exposure. Stimulation for 5 to 10 min increased ERK1/2 phosphorylation (fourfold vs control, p<0.05). The effect of C-peptide was transient, and the level of ERK1/2 phosphorylation decreased gradually to the basal level after 30 to 60 min of stimulation (Fig. 1a). A physiological concentration of C-peptide (1 nmol/l) increased ERK1/2 phosphorylation approximately fourfold (P<0.05); no further effect was observed for concentrations of 3 and 10 nmol/l. Interestingly, a further increase in C-peptide concentration to 100 nmol/l resulted in only a half-maximal effect (Fig. 1b). Based on the obtained time- and concentration-dependent data, the stimulation conditions (5 nmol/l of C-peptide and 10 min of incubation) were chosen and used throughout the study. Under these experimental conditions, the C-terminal pentapeptide of C-peptide was found to exhibit similar effects to those of native C-peptide on ERK1/2 phosphorylation, while scrambled C-peptide had no effect (Fig. 1c). When cells were pretreated with PD98059 (an inhibitor of MEK1, a kinase upstream of ERK1/2), pertussis toxin (Gi protein inhibitor), and verapamil and nifedipine (L-type Ca2+ channel blockers), the effects of C-peptide and its C-terminal pentapeptide on phosphorylated ERK1/2 were abolished (Fig. 1d, e), suggesting that the effects of C-peptide are G-protein- and Ca2+-dependent. The effects of C-peptide on the phosphorylation of JNK are shown in Fig. 2a. C-peptide and the C-terminal pentapeptide, but not scrambled C-peptide, increased JNK phosphorylation approximately 2.7-fold (p<0.05). These effects were abolished by PTX (Fig. 2b), indicating that C-peptide stimulates JNK phosphorylation via Gi/Go-protein-coupled receptors. C-peptide had no effect on p38 MAP kinase phosphorylation.
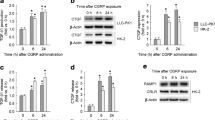
Effect of C-peptide, C-terminal pentapeptide, scrambled C-peptide, PD98059, pertussis toxin and Ca2+ channels blockers on ERK1/2 phosphorylation. Cells were serum starved overnight, stimulated with human C-peptide, and cell lysates were subjected to western blot analyses to determine ERK1/2 phosphorylation. Amount of phosphorylated ERK1/2 in the densitometric quantification is expressed as fold increase vs control. a Time-dependent effects of 5 nmol/l human C-peptide observed from 1 to 60 min. b Dose-dependent effects were tested in the range of 1 to 100 nmol/l in cultures incubated for 10 min. c Cells were stimulated with or without human C-peptide (CP), pentapeptide (PP) or scrambled C-peptide (ScrCP) at 5 nmol/l for 10 min. d Cells were pretreated overnight in the absence or presence of 100 ng/ml pertussis toxin (PTX), or 20 μmol/l PD98059 for 20 min or (e) 20 μmol/l nifedipine (nif) or 20 μmol/l verapamil (ver) for 20 min in serum-free media. Cells were then stimulated with 5 nmol/l C-peptide or pentapeptide for 10 min (d, e). Results are means ± SE for six independent experiments. *p<0.05 vs control. A representative western blot image is shown in the upper panel of each graph
Effect of C-peptide on JNK phosphorylation. Cells were stimulated with 5 nmol/l human C-peptide (CP), pentapeptide (PP) and scrambled C-peptide (ScrCP) for 10 min. a Cells were also pretreated overnight in the absence or presence of 100 ng/ml pertussis toxin (PTX). The cells were then stimulated with 5 nmol/l C-peptide and ScrCP for 10 min. b Results are means ± SE for five independent experiments. *p<0.05 vs. control. A representative western blot image is shown in the upper panel of each graph
Effects of C-peptide on Akt/PKB phosphorylation
C-Peptide at 5 nmol/l had a robust effect on Akt phosphorylation (Fig. 3b), which peaked between 3 and 10 min of stimulation. Similar to its effects on ERK1/2 phosphorylation, the most marked effects of C-peptide were on Akt phosphorylation at concentrations close to physiological concentrations (Fig. 3b). The C-terminal pentapeptide was approximately equipotent with the native peptide in stimulating Akt. These effects were inhibited when the cells were pretreated with PTX (Fig. 3c). Scrambled C-peptide did not significantly stimulate Akt.
Effect of C-peptide on Akt phosphorylation. Cells were serum starved overnight and stimulated with human C-peptide. Total cell lysates were subject to western blot analyses to determine Akt phosphorylation. The amount of phosphorylated Akt in the densitometric quantification is expressed as fold increase vs control. Results are means ± SE for three to four independent experiments. *p<0.05 vs control. A representative western blot image is shown in the upper panel of each graph. a Time-dependent effects of 5 nmol/l human C-peptide observed from 1 to 30 min. b Dose-dependent effects were tested by increasing the concentration of C-peptide from 1 to 100 nmol/l in cultures incubated for 10 min. c Effects of C-terminal pentapeptide, scrambled human C-peptide and pertussis toxin (PTX) treatment on C-peptide-stimulated Akt phosphorylation
Effects of C-peptide on PKC activation
PKC isoform-specific antibodies (anti-PKC-α, -β, -γ, -δ, -ɛ, -ζ, -η and -θ) were used to detect the effects of C-peptide on the translocation of different isoforms of PKC from the cytosol to cell membranes in HRTC. The results show that C-peptide specifically increases the abundance of PKC-δ and -ɛ in the membrane fractions 2.5- and 3.1-fold (p<0.05), respectively (Fig. 4a, b), while isoforms α, γ, ζ and θ remain unchanged (data not shown). The isoforms β and η were barely detectable in the HRTC membrane fractions. The C-terminal pentapeptide was found to be equipotent with the full-length C-peptide with regard to PKC translocation. Scrambled C-peptide had no effect. PMA, used as a positive control, increased the translocation of isoforms α, γ, δ and ɛ. Insulin translocates PKC-ζ but not the other isoforms (data not shown). When the cells were pretreated with rottlerin, a PKC-δ-specific inhibitor, the effect of C-peptide on PKC-δ, but not on PKC-ɛ, was abolished (Fig. 4c, d). When using 1 μmol/l of GF109203X, the broad-range PKC inhibitor, the effects of C-peptide on the isoforms δ and ɛ were inhibited. To further confirm the activation effect of C-peptide on PKC-δ, a phospho-specific antibody to PKC-δ was used. C-Peptide was found to increase PKC-δ phosphorylation in total cell lysates and this effect was abolished by PTX (Fig. 5).
Effects of C-peptide on the activation of PKC isoforms. Cells were serum starved overnight, pretreated without (a, b) or with (c, d) 1 μmol/l GF109203X or 20 μmol/l rottlerin (Rot) for 30 min, and then incubated with 5 nmol/l C-peptide, pentapeptide or scrambled C-peptide, or with 100 nmol/l insulin or PMA for 10 min. PKC-δ and PKC-ɛ were detected by western blotting of membrane fractions with isoform-specific anti-PKC antibodies. Results are means ± SE for eight independent experiments. *p<0.05 vs control. A representative western blot image is shown in the upper panel of each graph
Effect of pertussis toxin on C-peptide-induced phosphorylation of PKC-δ. Cells were pretreated overnight in the absence or presence of 100 ng/ml pertussis toxin in serum-free media. Cells were then stimulated with 5 nmol/l C-peptide or scrambled C-peptide for 10 min. Total cell lysates were subject to western blot analysis to determine PKC-δ phosphorylation. Results are means ± SE for four independent experiments. *p<0.05 vs control. A representative western blot image is shown in the upper panel of the graph
Effects of PKC inhibitors rottlerin and GF109203X on ERK1/2 and JNK phosphorylation
To determine whether the effects of C-peptide on ERK1/2 and JNK phosphorylation are mediated via a PKC-dependent pathway, a broad-spectrum PKC inhibitor, GF109203X, and the PKC-δ-specific inhibitor rottlerin were used. The results show that both GF109203X (Fig. 6a) and rottlerin (Fig. 6b) inhibit the effect of C-peptide on the ERK1/2 phosphorylation. JNK phosphorylation by C-peptide was also inhibited by rottlerin (Fig. 6c).
Effects of PKC inhibitors on C-peptide-induced phosphorylation of ERK1/2 and JNK. Cells were pretreated in the absence or presence of 1 or 10 μmol/l GF109203X (GFX) (a), or 20 μmol/l rottlerin (Rot) (b, c) for 30 min in serum-free media. Cells were then stimulated with 5 nmol/l C-peptide for 10 min. Total cell lysates were subjected to western blot analysis to determine ERK1/2 phosphorylation. Results are means ± SE for six independent experiments. *p<0.05 vs control. A representative western blot image is shown in the upper panel of each graph
Effects of phospholipase C inhibition on phosphorylation of ERK1/2, JNK and PKC-δ
The present results suggest that C-peptide stimulation of ERK1/2 and JNK is dependent on the PKC isoforms PKC-δ and -ɛ. Activation of PKC requires diacylglycerol, which is a product of the hydrolysis of membrane inositol phospholipids by phospholipase C. In order to test the phospholipase C dependency of the C-peptide signal, cells were pretreated with the phospholipase C inhibitor U73122 prior to C-peptide stimulation. The results show that C-peptide-induced phosphorylation of ERK1/2, JNK and PKC-δ (Fig. 7a, b, d) is inhibited by U73122.
Effects of phospholipase C inhibition on C-peptide-induced phosphorylation of ERK1/2 (a), JNK (b), p38 MAP kinase (c) and PKC-δ (d). Cells were serum starved overnight, and pretreated in the absence or presence of 10 μmol/l phospholipase C inhibitor U73122 for 30 min in serum-free media. Cells were then stimulated with 5 nmol/l C-peptide for 10 min. Total cell lysates were subject to western blot analysis to determine the phosphorylation of ERK1/2, JNK, p38 MAP kinase and PKC-δ. Results are means ± SE for five independent experiments. *p<0.05 vs control. A representative western blot image is shown in the upper panel of each graph
Effects of C-peptide on RhoA activation
A possible link between activation of PKCs and MAP kinases could be a PKC-dependent activation of small GTPases [29, 30]. Indeed, as shown in Fig. 8a, C-peptide was found to promote the translocation of small GTPase RhoA from the cytosol to the membrane fraction (3.5-fold increase; p<0.05). This effect was inhibited by both the PKC-δ-specific inhibitor rottlerin and the broad-spectrum PKC inhibitor GF109203X. These results were confirmed in an experiment on the binding of C-peptide-activated RhoA to the GST-Rho binding domain of rhotekin (Fig. 8b). C-Peptide stimulates RhoA-rhotekin binding activity essentially to the same extent as the externally added nonhydrolysable GTP analogue GTPγS.
C-Peptide stimulation of RhoA is PKC dependent. Cells were serum starved overnight, pretreated without or with 1 μmol/l GF109203X or 20 μmol/l rottlerin for 30 min, and then incubated with 5 nmol/l C-peptide for 10 min. a Cells were scraped in sucrose buffer (see “Materials and methods”), and membrane fractions were obtained by ultracentrifugation. Membrane fractions were subject to western blot analysis to determine the RhoA activity. b Cells were lysed, and RhoA binding to the rhotekin-Rho binding domain agarose was assessed by western blotting. Results are means ± SE for six independent experiments. *p<0.05 vs control. A representative western blot image is shown in the upper panel of each graph
Discussion
Physiological and clinical effects of C-peptide in type 1 diabetes and its complications have been documented, but the molecular mechanism of C-peptide action still remains incompletely understood. A number of studies, performed on different cell lines, have provided evidence for broad cellular responses to C-peptide stimulation. Several key signalling molecules, such as PKC and the MAP kinase family members have been reported to be activated by C-peptide [3, 4, 7, 27], but a specific signal transduction pathway from a proposed C-peptide receptor to downstream effector molecules has not been established. The identification of a specific C-peptide signalling pathway would be helpful for our understanding of its role in ameliorating or preventing the development of long-term complications in diabetes [13–15, 20, 23]. Therefore, the aim of the present study was to establish the sequence of signalling events following C-peptide stimulation using a pharmacological approach based on specific stepwise inhibition.
The present results show that C-peptide in the physiological concentration range stimulates two distal components of the MAP kinase signalling pathway, ERK1/2 and JNK, in human renal tubular cells. The effect of C-peptide was concentration dependent and transient, showing rapid kinetics. An increase in JNK phosphorylation following exposure of cells to C-peptide has not been reported previously. The C-terminal pentapeptide of C-peptide was found to be equipotent with the full-length C-peptide, in accordance with previous findings for the stimulation of Na+, K+-ATPase by this fragment [6, 15, 31, 32], whereas scrambled C-peptide had no effect. In contrast to a previous study involving mouse endothelial cells [27], we did not observe an increase in p38 MAP kinase phosphorylation, which may be related to tissue- and species-specific differences. C-Peptide stimulation also led to a rapid and transient increase in the phosphorylation of Akt (protein kinase B). Akt stimulation required a similar concentration of C-peptide to ERK1/2 phosphorylation. This effect was sensitive to pertussis toxin, indicating that Akt activation by C-peptide occurs via a G-protein-mediated signal. In contrast, exposure of human renal tubular cells to high nonphysiological concentrations of C-peptide does not result in activation of Akt or MAP kinase. This may be a consequence of the rapid desensitisation of the C-peptide pathway, or it may be due to the activation of another cell signalling system, masking the low concentration effect of C-peptide.
The nature of the C-peptide binding site has not yet been reported. However, consistent with previous studies from this laboratory and others [1–3, 6], all C-peptide-modulated signalling events were inhibited by pertussis toxin, indicating that the signal is mediated via a Gi- or Go-protein. The observed stimulation of the PI-3 kinase and Akt signalling pathway is not necessarily contradictory to the proposed G-protein involvement. Stimulation of Gi/Go-protein-coupled receptors may result in activation of PI-3 kinase-γ via its association with the dissociated βγ subunits of the G-protein complex [33, 34]. Notably, PI-3 kinase-γ is highly expressed in kidney proximal tubular cells [35]. Alternatively, C-peptide may conceivably activate PI-3 kinase via stimulation of the insulin receptor tyrosine kinase or via attenuation of tyrosine phosphatase activity [36]. In contrast, human skeletal muscle strips exposed to C-peptide respond with augmented glucose uptake, but fail to show phosphorylation of the insulin receptor or its tyrosine kinase activity [37]. Likewise, studies of C-peptide binding to cell membranes show that bound C-peptide cannot be displaced by insulin, nor can bound insulin be displaced by C-peptide [1]. The latter findings suggest that C-peptide and insulin bind to different sites, probably on different receptors, but the possibility of there being two different loci on the same receptor cannot be excluded.
Previous reports indicate that C-peptide stimulation of ERK1/2 may be abolished by downregulation of PKC expression [3]. C-peptide induces PKC-α translocation to the membrane fraction in rat medullary thick ascending limb tubular cells [7] and activates PKC-α in opossum kidney tubular cells [26]. In contrast to the latter study, we found that in human renal tubular cells, C-peptide causes translocation of the PKC isoforms δ and ɛ to the membrane fraction, while the cellular distribution of the PKC isoforms α, γ, ζ and θ was not influenced by C-peptide. The broad-spectrum PKC inhibitor GF109203X abolished C-peptide activation of both PKC-δ and -ɛ, while the specific PKC-δ inhibitor rottlerin abolished only PKC-δ activation. Furthermore, both rottlerin and GF109203X abolished the effect of C-peptide on ERK1/2 phosphorylation. Thus, C-peptide in human tubular cells activates ERK1/2 via PKC-δ.
Since the effect of C-peptide on ERK phosphorylation was transient, it was of interest to show whether desensitisation of the signalling pathway occurs at the PKC level. We incubated human renal tubular cells with 1 and 5 nmol/l of C-peptide for 24 h and assessed the expression level of different PKC isoforms. However, levels of expression of Ca2+-and-phospholipid-sensitive PKC isoforms α and γ, as well as C-peptide-regulated diacylglycerol-sensitive PKC isoforms δ and ɛ, were not affected by long-term C-peptide incubation (data not shown). We believe that the effect of C-peptide on ERK phosphorylation is transient due to the desensitisation of the C-peptide receptor/binding site. In addition, subsequent activation of protein phosphatases cannot be excluded.
C-peptide stimulation of PKC-δ, ERK1/2 and JNK phosphorylation was completely abolished by U73122, a specific phospholipase C inhibitor. Stimulation of phospholipase C by G-protein-coupled receptors leads to increases in intracellular diacylglycerol concentration [38]. Notably, PKC-δ and -ɛ belong to the subfamily of novel PKC, which requires only diacylglycerol for activation [39, 40]. Thus, we hypothesise that PKC-δ may act on MAP kinase phosphorylation following C-peptide exposure. At the same time, activated PKC-δ and -ɛ could phosphorylate and activate other downstream target proteins such as Ca2+ channels [41, 42]. L-Type Ca2+ channels have been reported to be positively modulated by novel PKC isoforms [41, 43] and it is established that C-peptide is capable of eliciting increases in intracellular Ca2+concentration [2, 5, 6]. In the present study we found that the Ca2+ channel blockers verapamil and nifedipine abolished the effect of C-peptide on ERK1/2 phosphorylation, indicating that C-peptide stimulation results in an influx of Ca2+ rather than in the release of intracellular Ca+2 stores. However, there was no activation of the Ca2+-sensitive PKC isoforms after C-peptide stimulation. Despite this, increases in Ca2+ could still be of importance for the facilitation of MAP kinase cascade activation [44]. Increases in Ca2+ may also modulate Ca2+-dependent protein phosphatase activity, which has been reported to be involved in C-peptide intracellular signalling [6]. In addition, C-peptide stimulation caused PKC-δ-dependent translocation of small GTPase RhoA from the cytosol to the cell membrane. This could provide a link between PKC phosphorylation and the activation of the MAP kinase signalling cascade, since the Rho/ras small GTPase family members have been implicated in the activation of JNK and ERK1/2 [29, 45].
It has been suggested that C-peptide plays an important role in the maintenance of vascular homeostasis via its effects on eNOS [8]. There is also evidence to indicate that C-peptide participates in the control of renal Na+, K+-ATPase activity, thereby contributing to the regulation of tubular sodium handling during postprandial periods [6, 7]. However, the precise role of C-peptide in the modulation of intracellular signalling in health or in diabetes is not fully understood. Taking into account our present finding of C-peptide activation of PKCs, ERK and JNK in human renal tubular cells, as well as previous findings reporting anti-apoptotic and proliferative [18] effects of C-peptide, a specific role of C-peptide in the protection against diabetic nephropathy can be envisaged. Loss of proximal tubular cells during the development of type 1 diabetic nephropathy may reflect severe defects in the proliferation and/or survival programs of these cells, possibly related to lack of periodical stimulation by C-peptide and to activation of downstream targets. Activation of MAP kinases is reported to result in cell proliferation [45], and an anti-apoptotic role of Akt/PKB stimulation has been demonstrated [46]. Subsequent activation of PKC and RhoA may also lead to Na+, K+-ATPase stimulation [6, 47], a well-established effect of C-peptide in renal tubular cells [6, 7, 31]. Thus, signals transmitted by C-peptide are likely to play a crucial role in maintaining the number and function of proximal tubular cells.
In conclusion, C-peptide signal transduction involves the following: (1) the stimulation of a pertussis-toxin-sensitive membrane binding site; (2) a Gi/Go-protein-coupled membrane receptor (probable involvement); (3) activation of phospholipase C, PKC-δ and -ɛ, and the small GTPase RhoA; and (4) phosphorylation of ERK1/2 and JNK (Fig. 9). When activated, ERK and JNK could phosphorylate transcription factors and downstream kinases responsible for chromatin remodelling, gene expression and cell proliferation. A parallel activation of Akt/PKB via PI-3 kinase-γ by the dissociated βγ subunits of G-protein could also occur, followed by subsequent activation of anti-apoptotic pathways in kidney cells, or activation of glycogen synthesis and glucose transport in muscle cells. The identification of the molecular mechanism of C-peptide action will bring new understanding of the physiological role of C-peptide and its therapeutic effects for the treatment of diabetes mellitus, and may facilitate the identification of a putative C-peptide receptor.
Schematic representation of intracellular signalling pathways for C-peptide. For comments see “Discussion”
Abbreviations
- eNOS:
-
endothelial nitric oxide synthase
- ERK:
-
extracellular-signal-regulated kinase
- HRTC:
-
human renal tubular cells
- JNK:
-
c-Jun N-terminal kinase
- MAP:
-
mitogen-activated protein
- PKC:
-
protein kinase C
- PMA:
-
phorbol myristate acetate
- PTX:
-
pertussis toxin
References
Rigler R, Pramanik A, Jonasson P et al (1999) Specific binding of proinsulin C-peptide to human cell membranes. Proc Natl Acad Sci U S A 96:13318–13323
Shafqat J, Juntti-Berggren L, Zhong Z et al (2002) Proinsulin C-peptide and its analogues induce intracellular Ca2+ increases in human renal tubular cells. Cell Mol Life Sci 59:1185–1189
Kitamura T, Kimura K, Okamoto S et al (2001) Proinsulin C-peptide rapidly stimulates mitogen-activated protein kinases in Swiss 3T3 fibroblasts: requirement of protein kinase C, phosphoinositide 3-kinase and pertussis toxin-sensitive G-protein. Biochem J 355:123–129
Grunberger G, Qiang X, Li Z et al (2001) Molecular basis for the insulinomimetic effects of C-peptide. Diabetologia 44:1247–1257
Wallerath T, Kunt T, Forst T et al (2003) Stimulation of endothelial nitric oxide synthase by proinsulin C-peptide. Nitric Oxide 9:95–102
Ohtomo Y, Aperia A, Sahlgren B, Johansson B-L, Wahren J (1996) C-peptide stimulates rat renal tubular Na+, K+-ATPase activity in synergism with neuropeptide Y. Diabetologia 39:199–205
Tsimaratos M, Roger F, Chabardès D et al (2003) C-Peptide stimulates Na, K-ATPase activity via PKC alpha in rat medullary thick ascending limb. Diabetologia 46:124–131
Kitamura T, Kimura K, Makondo K et al (2003) Proinsulin C-peptide increases nitric oxide production through mitogen-activated protein kinase-dependent transcriptional enhancement of endothelial nitric oxide synthase in rat aortic endothelial cells. Diabetologia 46:1698–1705
Zochodne DW, Verge VM, Cheng C et al (2000) Nitric oxide synthase activity and expression in experimental diabetic neuropathy. J Neuropathol Exp Neurol 59:798–807
Greene DA, Yagihashi S, Lattimer SA, Sima AA (1984) Nerve Na+–K+-ATPase, conduction, and myo-inositol in the insulin-deficient BB rat. Am J Physiol 247:E534–E539
Kjeldsen K, Braendgaard H, Sidenius P, Larsen J, Norgaard A (1987) Diabetes decreases Na+–K+ pump concentration in skeletal muscle, heart ventricular muscle, and peripheral nerves of rat. Diabetes 36:842–848
Wald H, Scherzer P, Rasch R, Popovtzer M (1993) Renal tubular Na+–K+-ATPase in diabetes mellitus: relationship to metabolic abnormality. Am J Physiol 265:F96–F101
Johansson B-L, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren J (2000) Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with type I diabetes. Diabet Med 17:181–189
Ekberg K, Brismar T, Johansson B-L, Jonsson B, Lindström P, Wahren J (2003) Amelioration of sensory nerve dysfunction by C-peptide in patients with type 1 diabetes. Diabetes 52:536–541
Sima AA, Zhang W, Sugimoto K et al (2001) C-Peptide prevents and improves chronic type 1 diabetic neuropathy in the BB/Wor rat. Diabetologia 44:889–897
Pierson C, Zhang W, Sima A (2003) Proinuslin C-peptide replacement in type 1 diabetic BB/Wor-rats prevents deficits in nerve fiber regeneration. J Neuropathol Exp Neurol 62:765–779
Li Z, Zhang W, Sima A (2002) C-Peptide prevents hippocampal apoptosis in type 1 diabetes. Int J Exp Diabetes Res 3:241–245
Li Z, Zhang W, Sima A (2003) C-Peptide enhances insulin-mediated cell growth and protection against high glucose-induced apoptosis in SH-SY5Y cells. Diabetes Metab Res Rev 19:375–385
Samnegård B, Jacobson S, Jaremko G, Johansson B-L, Sjöquist M (2001) Effects of C-peptide on glomerular and renal size and renal function in diabetic rats. Kidney Int 60:1258–1265
Johansson B-L, Kernell A, Sjöberg S, Wahren J (1993) Influence of combined C-peptide and insulin administration on renal function and metabolic control in diabetes type 1. J Clin Endocrinol Metab 77:976–981
Johansson B-L, Pernow J, Wahren J (2003) C-Peptide increases forearm blood flow in patients with type 1 diabetes via a nitric oxide dependent mechanism. Am J Physiol Endocrinol Metab 285:E864–E870
Hansen A, Johansson B-L, Wahren J, Bibra von H (2002) C-Peptide exerts beneficial effects on myocardial blood flow and function in patients with type 1 diabetes. Diabetes 51:3077–3082
Johansson B-L, Sundell J, Ekberg K et al (2004) C-Peptide improves adenosine-induced myocardial vasodilation in type 1 diabetes patients. Am J Physiol Endocrinol Metab 286:E14–E19
Pearson G, Robinson F, Beers Gibson T et al (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–158
Toker A (1998) Signaling through protein kinase C. Front Biosci 3:D1134–D1147
Al-Rasheed N, Meakin F, Royal E et al (2004) Potent activation of multiple signalling pathways by C-peptide in opossum kidney proximal tubular cells. Diabetologia 47:987–997
Kitamura T, Kimura K, Jung B et al (2002) Proinsulin C-peptide activates cAMP response element-binding proteins through the p38 mitogen-activated protein kinase pathway in mouse lung capillary endothelial cells. Biochem J 366:737–744
Söderhäll M, Bergerheim U, Jacobson S et al (1997) Molecular evidence for pap-G specific adhesion of Escherichia coli to human renal cells. J Urol 157:346–350
Lopez-Ilasaca M (1998) Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochem Pharmacol 3:269–277
Kranenburg O, Moolenaar W (2001) Ras-MAP kinase signaling by lysophosphatidic acid and other G protein-coupled receptor agonists. Oncogene 13:1540–1546
Ohtomo Y, Bergman T, Johansson B-L, Jörnvall H, Wahren J (1998) Differential effects of proinsulin C-peptide fragments on Na+, K+-ATPase activity of renal tubule segments. Diabetologia 41:287–291
Ido Y, Vindigni A, Chang K et al (1997) Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science 277:563–566
Stoyanov B, Volinia S, Hanck T et al (1995) Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science 269:690–693
Toker A, Cantley L (1997) Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature 6634:673–676
Bernstein H, Keilhoff G, Reiser M, Freese S, Wetzker R (1998) Tissue distribution and subcellular localization of a G-protein activated phosphoinositide 3-kinase. An immunohistochemical study. Cell Mol Biol (Noisy-le-grand) 44:973–983
Li Z, Qiang X, Sima A, Grunberger G (2001) C-Peptide attenuates protein tyrosine phosphatase activity and enhances glycogen synthesis in L6 myoblasts. Biochem Biophys Res Commun 280:615–619
Zierath J, Handberg A, Tally M, Wallberg-Henriksson H (1996) C-Peptide stimulates glucose transport in isolated human skeletal muscle independent of insulin receptor and tyrosine kinase activation. Diabetologia 39:306–313
Rhee S, Bae Y (1997) Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem 272:15045–15048
Newton A (1995) Protein kinase C: structure, function and regulation. J Biol Chem 270:28495–28498
Hofmann J (1997) The potential for isoenzyme-selective modulation of protein kinase C. FASEB J 11:649–669
Keef K, Hume J, Zhong J (2001) Regulation of cardiac and smooth muscle Ca(2+) channels (Ca(V)1.2a, b) by protein kinases. Am J Physiol Cell Physiol 281:C1743–C1756
Kamp T, Hell J (2000) Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res 87:1095–1102
Hantash B, Thomas A, Reeves J (2000) Dual effect on protein kinase C on L-type calcium activity in L6 cells. Biophys J 78:203A
Agrell N, Bachs O, Rocamora N, Villalonga P (2002) Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal 14:649–654
Gutkind J (2000) Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci Signal Transduct Knowl Environ 40:RE1
Lawlor M, Alessi D (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114:2903–2910
Lecuona E, Ridge K, Pesce L, Batlle D, Sznajder JI (2003) The GTP-binding protein RhoA mediates Na, K-ATPase exocytosis in alveolar epithelial cells. Mol Biol Cell 14:3888–3897
Acknowledgements
We thank J. R. Zierath for helpful discussions. This work was supported by grants from the Swedish Medical Research Council, the Swedish Heart and Lung Foundation, the Novo-Nordisk Foundation, the Swedish Society of Medicine and Creative Peptides Sweden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, Z., Davidescu, A., Ehrén, I. et al. C-peptide stimulates ERK1/2 and JNK MAP kinases via activation of protein kinase C in human renal tubular cells. Diabetologia 48, 187–197 (2005). https://doi.org/10.1007/s00125-004-1602-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1602-5