Abstract
Aims/hypothesis
Few data are available on lung dysfunction in children with diabetes. We studied the association of pulmonary function variables (flows, volumes and alveolar capillary diffusion) with disease-related variables in children with type 1 diabetes mellitus.
Methods
We studied 39 children with type 1 diabetes (mean age 10.9±2.6 years, disease duration 3.6±2.4 years, insulin·kg−1·day−1 0.77±0.31) and 30 healthy control children (mean age 10.4±3.0 years). Pulmonary function tests included spirometry, N2 wash-out and the single-breath diffusing capacity for carbon monoxide (DLCO) corrected for the alveolar volume (DLCO/VA). Glycaemic control was assessed on the basis of HbA1c, with HbA1c values of 8% or less considered to indicate good glycaemic control, and HbA1c values of 8% or more considered to indicate poor control.
Results
Children with poor glycaemic control had comparable percentage values for predicted flows and volumes but lower DLCO/VA values than children with good glycaemic control and healthy control children (86.7±12.6 vs 99.8±18.4 and 102.0±15.7; p<0.05). The predicted DLCO/VA percentages correlated with HbA1c levels (r=−0.39, p=0.013). A multiple regression analysis (stepwise model) controlling for HbA1c levels and other disease-related variables (age of disease onset, disease duration, daily insulin dose/kg, sex) identified HbA1c levels as the sole predictor of DLCO/VA in percent.
Conclusions/interpretation
In children with type 1 diabetes, the diffusing capacity diminishes early in childhood and is associated with poor metabolic control. Although low DLCO/VA levels in these children probably reflect pulmonary microangiopathy induced by type 1 diabetes, other factors presumably influencing CO diffusion capacity measurements (e.g. a left shift in HbA1c resulting in high O2 binding and low CO binding) could explain the apparent capillary and alveolar basal membrane dysfunction.
Similar content being viewed by others
Introduction
Diabetes mellitus is associated with metabolic and microvascular abnormalities as well as multi-organ and multisystem dysfunction [1]. Pulmonary damage in diabetic patients arises from several mechanisms, including biochemical changes in connective tissue, especially in collagen and elastin [2, 3]. Non-enzymatic protein glycosylation induced by chronic hyperglycaemia has been proposed as one of the determinant mechanisms leading to diabetic microangiopathy [4, 5]. Owing to its abundant connective tissue and diffuse microvascular circulation, the lung is thought to be a target organ for diabetic disease [6]. Because pulmonary function and gas exchange depend partly on the integrity of the connective tissue and microcirculation within the lung, changes involving these structural components could lead to mechanical lung dysfunction and impaired blood gas exchange [6, 7, 8]. Studies conducted in adult patients with type 1 diabetes report diminished elastic lung recoil [9, 10], reduced lung volumes [11, 12, 13, 14, 15] and altered alveolocapillary diffusion [12, 14, 16]. Various mechanisms have been proposed to explain reduced diffusing capacity, including alveolocapillary membrane thickening due to non-enzymatic glycosylation of lysine and hydroxyproline [5, 6] and decreased capillary blood-flow volume [10].
Because most published studies deal with adult and adolescent patients, few data are available on lung function in diabetic children. The few studies available in children report a mild decrease in vital capacity [17], decreased or normal forced vital capacity (FVC) [18, 19], slightly increased airway resistance [19, 20] and normal lung diffusion capacity for carbon monoxide (DLCO) corrected for alveolar volume (DLCO/VA) as compared with reference values [19]. None of these paediatric studies found a relationship between lung function and disease-related variables (age of disease onset, duration of disease, glycaemic control, daily insulin dose, proteinuria, retinopathy).
Our primary purpose in this study was to find out whether DLCO/VA values were lower in children with type 1 diabetes than in healthy age-matched control subjects. In the same patients, we also sought to establish whether an association exists between lung function variables (lung flows, volumes and DLCO/VA) and disease-related variables, and especially an association between low DLCO/VA and poor glycaemic control.
Subjects and methods
Subjects
For this study we selected 42 consecutive child patients with type 1 diabetes (23 boys; age range: 5–14 years), who attended our University paediatric outpatient clinic for periodic assessment of disease. All outpatients were insulin-dependent when studied and receiving insulin therapy (0.77±0.31 insulin·kg−1·day−1 in 3 doses). None of the children smoked. When recruited for the study, none of the participants manifested clinical signs or symptoms of diabetic neuropathy, autoimmune disease, kidney or ocular disease. As a control group we studied 30 healthy, age-matched children, without lung disease, attending our clinic for general clinical evaluation. None of the subjects had suffered acute respiratory infections in the previous three weeks. All participants’ parents provided written informed consent to the study. The study procedures were approved by the hospital ethics committee.
Study protocol
A questionnaire was used to build up a detailed personal and family history of cardiorespiratory illnesses. Subjects were also clinically evaluated at the time of testing to exclude acute airways disease. Before pulmonary testing, all subjects had blood samples taken for estimation of haemoglobin concentrations and HbA1c. Microalbuminuria was measured from urine samples collected over 24 hours. Lung testing started with spirometry, followed by the N2-washout procedure. Subjects were then allowed to rest for at least 20 minutes before DLCO measurements.
To test lung dynamic and static volumes, flows and DLCO, we used an ALTAIR 4000 COSMED apparatus (Cosmed, Rome, Italy). Pneumotachographs and gas analysers were calibrated daily. Pneumotachographs were tested at several inspiratory and expiratory flows (by pumping in and out) with a 3-L calibration syringe. Gas analysers were automatically calibrated as follows: (i) the electric signal from the N2 analyser was set to zero by switching off the ionisation chamber, the analyser was calibrated for electric gain by re-starting the ionisation chamber and adjusting the measure of ambient N2 level (≅77–79%) to ambient temperature pressure saturation conditions; (ii) the aspiration pump was calibrated with a certified 100% O2 gas cylinder; and (iii) the zero signal for CO and He was calibrated with ambient air, then calibrated for the electric gain with certified gas mixtures (see below).
To ensure body temperature pressure saturation conditions, we used heated pneumotachographs for the spirometric measurements. Variables recorded were forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), mean forced expiratory flow during the middle half of the FVC (FEF25–75%) as recommended [21]. We also measured the maximal expiratory flow at 25% of the FVC (MEF25%). Functional residual capacity (FRC) was measured by the open-circuit method of N2 washout [22]. Gas cylinders containing certified 100% O2 were used for N2-washout measurements. DLCO was measured by a standardised single-breath method [23]. The inhalation gas mixture contained 0.3% CO, 10% helium and balance air [23]. Duplicate DLCO measurements differing within 10% of each other were obtained; the average of these two measurements was reported. DLCO values were corrected for individual haemoglobin concentrations [23] and alveolar volume, and were expressed as DLCO/VA. Inspiratory volumes were considered acceptable when subjects achieved an inspired volume above 90% of their vital capacity in all tests. All spirometric, N2 wash-out and DLCO data are reported as percentages of the normal predicted values by age, height and sex [24]. Glycaemic control in patients was evaluated by high-performance liquid chromatography (DIAMAT, Bio-rad, Munich, Germany) of HbA1c at pulmonary function testing and every three months during the year preceding the study. We considered HbA1c values of 8% or less to indicate good glycaemic control and HbA1c values of more than 8% to indicate poor control. We also evaluated diabetic patients according to the duration of disease (years), age at onset of disease, and insulin dose (insulin·kg−1·day−1). Patients were screened for limited joint mobility as defined by Rosenbloom and co-workers [25], and for other diabetic complications by clinical ophthalmoscopy, clinical neurological examination, creatine clearance, and microalbuminuria measured over 24 hours (detected by γ-counter radioimmunoassay). Microalbuminuria was defined as an albumin excretion rate greater than 25 µg/min in at least two or three consecutive urine samples collected over 24 h.
Statistical analysis
The Kolmogorov–Smirnov goodness-of-fit test was used to ascertain normal distribution of all variables. Data are expressed as means ± SD. Coefficients of reproducibility of duplicated measurements were calculated as twice the standard deviation (SD) of normally distributed data. Unpaired t test or ANOVA with post hoc Scheffe test were used for comparisons between two or more subgroups as needed. Pearson coefficient correlations between variables were determined. Significant correlated disease-related variables and selected clinical variables were then included in a stepwise linear multiple-regression model using DLCO/VA%, FEV1%, FEF25–75% or MEF25% separately as dependent variables against disease-related variables. We considered p values of less than 0.05 to be statistically significant. Statistical analysis was done with SPSS PC (version 9 for Windows).
Results
Of the 42 patients initially enrolled, 39 met the inclusion criteria and completed both DLCO and HbA1c measurements. All 69 participants (39 patients and 30 control subjects) had anthropometric characteristics within the normal range (Table 1). When tested, none of the participants had clinical symptoms of acute or active disease and all of them had FEV1 values above 80% of the predicted values. None of the patients showed signs of low joint mobility or had ophthalmoscopic findings indicative of retinopathy. In three patients, fluorescein angiography was done to investigate ophthalmoscopic findings of doubtful ocular fundus, and yielded a diagnosis of non-proliferative retinopathy. None of the 39 patients had abnormal renal function; only two had microalbuminuria (>25 mg/day); and 19 had poor glycaemic control (HbA1c >8%).
For technical reasons two patients were unable to complete DLCO measurements. No HbA1c values were available for one patient, who was a new-onset patient.
Coefficients of reproducibility of duplicated DLCO measurements (2 SD) were within the acceptable limits [23]: 8.2% for healthy control children and 8.6% for diabetic children (children with good glycaemic control: 8.4%; children with poor glycaemic control: 9.0%). Pulmonary function testing showed comparable lung flows and volumes but lower DLCO/VA values in the 39 diabetic patients than in the 30 healthy controls (93.0±18.2 vs 102.0±15.7 percent of predicted value; p=0.03 by unpaired t test). DLCO/VA values were lowest in patients with poor glycaemic control (Table 1). Among the diabetic patients, boys had lower percent FEF25–75 and MEF25 than girls (mean FEF25–75: 95.9±19.9 vs 112.6±21.7, p=0.014; data not shown), but similar FEV1 and FVC values. The two subjects with microalbuminuria (>25 mg/24 h) had DLCO/VA values within the normal range (105%, 112%). The three patients who underwent fluorescein angiography all had grade 1 retinopathy and low DLCO/VA values (66%, 68% and 73%).
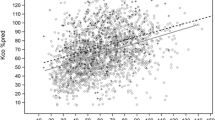
Percentages of predicted FEV1, FEF25–75 and MEF25 correlated with the age at diabetes onset (FEV1: r=0.37, p=0.023; FEF25–75: r=0.47, p=0.003; MEF25: r=0.44, p=0.004), but not with disease duration or HbA1c or daily insulin dose/kg or anthropometric characteristics. Conversely, percentage of the predicted DLCO/VA value did correlate with HbA1c levels (r=−0.39, p=0.013), but not with the other disease-related variables (Fig. 1). There was no correlation between static lung volumes (percent of predicted FRC and TLC) and disease-related or anthropometric variables.
Stepwise linear multiple-regression models investigating each of the correlated lung-function variables (percent of predicted FEV1, FEF25–75, MEF25 and DLCO/VA) separately as dependent variables, and age of disease onset, disease duration, insulin dose/kg, HbA1c levels and sex as independent variables added no other correlated independent variables, except for FEF25–75 and MEF25, which can both be explained by age of disease onset and male sex (data not presented).
Discussion
In this study of 10-year-old children with type 1 diabetes, we found that although the diabetic subjects had normal pulmonary function, they nevertheless had reduced DLCO/VA values. This was especially true of the children with poor glycaemic control. Our study therefore extends the existing knowledge on reduced DLCO in adult patients with type 1 diabetes [12, 13, 14, 15, 16], suggesting that this lung function index starts to decline in childhood.
The only previous study, to our knowledge, to measure DLCO/VA in diabetic children found no relationship between DLCO/VA and HbA1c levels [19]. The inverse relationship between DLCO/VA and HbA1c values observed in our children is nevertheless consistent with previous findings in adults, showing that diabetic patients with poor long-term metabolic control had lower DLCO values than comparable patients with long-term near-normoglycaemia [26]. We found the lowest DLCO/VA values in the three children who had initial retinopathy. Previous studies in adults have shown reduced DLCO in subjects with diabetic retinopathy and these changes correlated with disease duration [14, 27]. Weir and co-workers have shown an association between alveolocapillary membrane changes and ocular microangiopathy (retinopathy or maculopathy) [27]. Although kidney damage was not yet manifest in our young patients, and histologically undocumented, their low DLCO/VA could raise the possibility of early lung microangiopathy. A low DLCO/VA does not, however, necessarily mean damage to the alveolocapillary membrane. Because CO uptake is a measure of gas transfer across the alveolar epithelial layer and capillary surface area and function, DLCO values depend on multiple physiological variables [23]. Theoretically, reduced CO uptake reflects several variables, including pulmonary blood flow, pulmonary capillary surface area, lung volume, and lung parenchymal integrity. Studies in diabetic adults suggest that CO uptake diminished through two main mechanisms: reduced pulmonary blood flow [10, 28], and thickened basal membranes of the alveolar and capillary walls [4, 28, 29]. An alternative explanation for the diminished DLco/VA we found in children with type 1 diabetes is that glycosylated haemoglobin might have been left-shifted [30, 31]. Children might therefore have had low DLco levels because high oxygen binding lowered CO binding. This biochemical mechanism would also plausibly explain why patients with the highest HbA1c had the lowest DLCO.
Published data on lung mechanics in diabetic subjects are conflicting. Some reports describe decreased volumes and flows in diabetic adults [12, 13, 14, 15], adolescents [9, 11] and children [17, 18] as compared with control subjects or reference values. Others, conversely, report normal lung volumes and flows in diabetic adults [8, 16, 32, 33], adolescents [9, 34] and children [19].
Although some studies in adolescents and young adults with type 1 diabetes have described a loss of elastic recoil [9, 10] and also decreased total lung capacity [9], another study found neither to be changed [34]. Others report a restrictive pattern in patients with limited joint mobility, attributing reduced pulmonary function to stiffness and thickening of the skin and connective tissue within the lung parenchyma [11]. These observations suggest that altered lung mechanics in diabetic subjects could arise from damage to lung connective tissue (collagen, elastin) induced by non-enzymatic glycosylation [6].
None of the children we studied had reduced static lung volumes or limited joint mobility. Their dynamic volumes and flows were within the predicted normal range. However, the earlier their disease onset, the lower their values tended to be, supporting previous observations from our research group [35, 36]. Earlier onset of diabetes could allow the respiratory system to enact distinct adaptive mechanisms that could remain stable over the years. After following children with mildly reduced FVC for 3 years, Primhak and colleagues found that FVC values remained stable. Hence they concluded that “a tendency toward reduced lung volumes exists in type 1 diabetes and may not be a direct result of the metabolic disturbance” [18].
In conclusion, children with type 1 diabetes and poor glycaemic control have lower DLCO/VA values (but similar flows and volumes) than their counterparts with good glycaemic control and healthy age-matched controls. Although diminished DLCO/VA does not confirm alveolocapillary membrane dysfunction, in the absence of more specific findings from non-invasive testing of lung damage, it should nevertheless raise the suspicion of disease complications related to poor glycaemic control in children with type 1 diabetes.
Abbreviations
- DLCO :
-
lung diffusion capacity for carbon monoxide
- DLCO/VA :
-
lung diffusion capacity for carbon monoxide corrected by alveolar volume
- FEF25–75 :
-
mean forced expiratory flow during the middle half of the FVC
- FEV1 :
-
forced expiratory volume in 1 second
- FRC:
-
functional residual capacity
- FVC:
-
forced vital capacity
- LJM:
-
limited joint mobility
- MEF25 :
-
maximal expiratory flow at 25% of the FVC
- TLC:
-
total lung capacity
References
Spiro RG (1976) Search for a biochemical basis of diabetic microangiopathy. Diabetologia 12:1–14
Sternberg M, Cohen-Forterre L, Peyroux J (1985) Connective tissue in diabetes mellitus. Biochemical alterations of the intercellular matrix with special reference to proteoglycans, collagens and basement membranes. Diabet Metab 11:27–50
Hamlin CR, Kohn RR, Luschin JH (1975) Apparent accelerated aging of human collagen in diabetes mellitus. Diabetes 24:902–904
Vracko R, Thorning D, Huang T W (1979) Basal lamina of alveolar epithelium and capillaries. Quantitative changes with aging and in diabetes mellitus. Am Rev Respir Dis 120:973–983
Vogt BW, Schleicher ED, Wieland OH (1982) ε-amino-lysine-bound glucose in human tissue obtained at autopsy. Diabetes 31:1123–1127
Sandler M (1990) Is the lung a “target organ” in diabetes mellitus? Arch Intern Med 150:1385–1388
Sandler M, Stewart RI, Gemperli BM, Hanekom C, Kuhn SH (1987) Serum alpha-1-protease inhibitor activity and pulmonary function in young insulin-dependent diabetic subjects. Respiration 52:281–289
Sandler M, Bunn AE, Stewart RI (1987) Cross-section study of pulmonary function in patients with insulin-dependent diabetes mellitus. Am Rev Respir Dis 135:223–229
Schuyler MR, Niewoehner DE, Inkley SR, Kohn R (1976) Abnormal lung elasticity in juvenile diabetes mellitus. Am Rev Respir Dis 113:37–41
Sandler M, Bunn AE, Stewart RI (1986) Pulmonary function in young insulin-dependent diabetic subjects. Chest 90:670–675
Schnapf BM, Banks LA, Silverstein JH, Rosenbloom AL, Chesrown SE, Loughlin GM (1984) Pulmonary function in insulin-dependent diabetes mellitus with limited joint mobility. Am Rev Respir Dis 130:930–932
Bell D, Collier A, Matthews DM, Cooksey EJ, McHardy GJ, Clarke BF (1988) Are reduced lung volumes in IDDM due to defect in connective tissue? Diabetes 37:829–831
Cooper BG, Taylor R, Alberti KGMM, Gibson GJ (1990) Lung function in patients with diabetes mellitus. Respir Med 84:235–239
Asunuma Y, Fujiya S, Ide H, Agishi Y (1985) Characteristics of pulmonary function in patients with diabetes mellitus. Diabetes Res Clin Pract 1:95–101
Innocenti F, Fabbri A, Anichini R et al. (1994) Indications of reduced pulmonary function in type I (insulin-dependent) diabetes mellitus. Diabetes Res Clin Pract 25:161–168
Isotani H, Nakamura Y, Kameoka K et al. (1999) Pulmonary diffusing capacity, serum angiotensin-converting enzyme activity and the angiotensin-converting enzyme gene in Japanese non-insulin-dependent diabetes mellitus patients. Diabetes Res Clin Pract 43:173–177
Buckingham B, Perejda AJ, Sandborg C, Kershnar AK, Uitto J (1986) Skin, joint and pulmonary changes in type 1 diabetes mellitus. Am J Dis Child 140:420–423
Primhak RA, Whincup G, Tsanakas JN, Milner RDG (1987) Reduced vital capacity in insulin-dependent diabetes. Diabetes 36:324–336
Van Gent R, Brackel HJ, De Vroede M, Van Der Ent CK (2002) Lung function abnormalities in children with type I diabetes. Respir Med 96:976–978
Verroti A, Verini M, Chiarelli F, Verdesca V, Misticoni G, Morgese G (1993) Pulmonary function in diabetic children with or without persistent microalbuminuria. Diabetes Res Clin Pract 21:171–176
American Thoracic Society (1995) Medical Section of American Lung Association. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med 153:1107–1136
Clausen JL, Coates AL, Quanjer PH (1997) Measurement of lung volumes in humans: review and recommendation from an ATS/ERS workshop. Eur Respir J 10:1205–1206
American Thoracic Society (1995) Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique—1995 update. Am J Respir Crit Care Med 152:2185–2198
Zapletal A, Samanek M, Paul T (1987) Lung function in children and adolescents: methods, reference values. Karger, Basel
Rosenbloom AL, Silverstein JH, Lezotte DC, Richardson K, McCallum M (1981) Limited joint mobility in childhood diabetes indicates increased risk for microvascular disease. N Engl J Med 305:191–194
Ramirez LC, Dal Nogare A, Hsia C et al. (1991) Relationship between diabetes control and pulmonary function in insulin-dependent diabetes mellitus. Am J Med 91:371–376
Weir DC, Jennings PE, Hendy MS (1988) Transfer factor for carbon monoxide in patients with diabetes with and without microangiopathy. Thorax 43:725–726
Niranjan V, McBrayer DG, Ramirez LC, Raskin P, Hsia CCW (1997) Glycemic control and cardiopulmonary function in patients with insulin-dependent diabetes mellitus. Am J Med 103:504–513
Weynand B, Jonckheere A, Frans A, Rahier J (1999) Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration 66:14–19
Ditzel J (1979) Changes in red cell oxygen release capacity in diabetes mellitus. Fed Proc 38:2484–2488
Bunn HF (1978) The glycosylation of haemoglobin: relevance to diabetes mellitus. Science 200:21–27
Oulhen PH, Barthetemy L, Bellet-Barthas M, Darragon T (1982) Respiratory function study on insulin-dependent diabetics (in French). Rev Fr Mal Respir 10:213–224
Fuso L, Cotroneo P, Basso S et al. (1996) Postural variations of pulmonary diffusing capacity in insulin-dependent diabetes mellitus. Chest 110:1009–1013
Schernthaner G, Haber P, Krummer R, Ludwing H (1977) Lung elasticity in juvenile onset diabetes mellitus. Am Rev Respir Dis 116:544–546
Villa MP, Bernardi F, Salardi S et al. (1988) Valutazione della funzionalità respiratoria nel bambino e nell’adolescente con diabete mellito di tipo 1. Riv Ital Ped 14:17–21
Villa MP, Cacciari E, Bernardi F, Cicognami A, Salazardi S, Zapulla F (1988) Bronchial reactivity in diabetic patients. Relationship to duration of diabetes and degree of glycemic control. Am J Dis Child 142:726–729
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villa, M.P., Montesano, M., Barreto, M. et al. Diffusing capacity for carbon monoxide in children with type 1 diabetes. Diabetologia 47, 1931–1935 (2004). https://doi.org/10.1007/s00125-004-1548-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1548-7