Abstract
Aims/hypothesis
Aldosterone blockade has followed in the footsteps of ACE inhibition in reducing mortality in patients with heart failure. This is associated with its beneficial effects on endothelial function and heart rate variability. Diabetes is another area, where angiotensin II withdrawal has proven to be of particular value. We postulated that aldosterone blockade with spironolactone might also have beneficial effects on the prognostic markers of endothelial function and heart rate variability in diabetic patients.
Methods
We assessed endothelial function by forearm venous occlusion plethysmography in 42 patients with Type 2 diabetes mellitus after 1 month of treatment with spironolactone or placebo allocated in a randomised double-blind trial. Of the 42 patients, 20 were on ACE inhibitor therapy. We also assessed heart rate variability, HbA1c and plasma angiotensin II levels at the end of each treatment period.
Results
Compared to placebo, spironolactone decreased forearm blood flow response to acetylcholine by 44.56±14.56% (p=0.003) in the group as a whole and by 57.61±15.56% (p<0.001) in the 20 patients on ACE inhibition. Spironolactone also worsened heart rate variability parameters, with root mean squared standard deviation decreased by 1.99±0.93 ms (p=0.03), low-frequency normalised power increased by 2.00±0.91 normalised units (nu) (p=0.03), high-frequency normalised power decreased by 1.98±0.94 nu (p=0.04) and the low frequency : high frequency ratio increased by 0.40±0.19 (p=0.04). HbA1c and angiotensin II increased during treatment with spironolactone by 0.26±0.07% (p=0.001) and 8.12±1.94 pg/ml (p=0.001) respectively.
Conclusions/interpretation
Spironolactone worsened endothelial function and heart rate variability in patients with Type 2 diabetes. These findings are possibly due to the worsening of glycaemic control and increase in plasma angiotensin II that were seen with spironolactone treatment. Thus the prescription of spironolactone to diabetic patients without heart failure does not seem to be justified.
Similar content being viewed by others
Introduction
Type 2 diabetes is associated with a high rate of cardiovascular events, and this remains so, even when glycaemic control is optimised [1]. It is therefore necessary to look to other strategies to improve cardiovascular prognosis in Type 2 diabetes.
It is well known that Type 2 diabetic patients suffer from endothelial dysfunction [2, 3] and poor heart rate variability [4, 5], both of which are adverse cardiovascular prognostic markers [6, 7, 8, 9, 10, 11, 12]. Angiotensin-converting enzyme inhibition improves both of these prognostic markers in diabetes [13, 14] and is also associated with an improvement in morbidity and mortality in diabetic subjects [15]. It is thought that ACE inhibitors beneficially affect these parameters by reducing angiotensin II bioactivity [16, 17]. However, ACE inhibition only poorly suppresses aldosterone, which is a cardiovascular culprit with the same adverse effect profile as angiotensin II [16].
In patients with heart failure ACE inhibitors have long been recognised to be beneficial. Now the RALES and EPHESUS trials have shown that adding an aldosterone blocker to an ACE inhibitor in these patients further reduces mortality [18, 19]. This is thought to be due to blockade of the main adverse cardiovascular effects of aldosterone, i.e. endothelial function and heart rate variability [20, 21]. We therefore wondered whether aldosterone blockade would produce similar beneficial effects in other situations where vascular dysfunction is prominent and where angiotensin II withdrawal has already proven to be of particular benefit. In the HOPE, LIFE and PROGRESS studies [15, 22, 23], angiotensin II blockade was most efficacious in subgroups of diabetic patients. This prompted us to investigate whether aldosterone blockade with spironolactone would improve endothelial function and heart rate variability in Type 2 diabetic subjects.
We chose to study endothelial function, because it is well known to be a good independent predictor of future cardiovascular risk [6, 7, 8, 9, 10, 11, 12], and because treatment-induced improvements in prognosis are usually mirrored by improvements in endothelial function [24, 25, 26], which is why endothelial function has been called a barometer of vascular health [27].
We also postulated that spironolactone might improve heart rate variability in this patient group, just as it does in patients with heart failure. Heart rate variability is known to be poor in diabetic patients, and has, like endothelial function, also been linked to cardiovascular prognosis [5, 28].
Subjects, materials and methods
Subjects
We recruited 42 Caucasian patients with Type 2 diabetes mellitus from the diabetes clinic at Ninewells Hospital and Medical School, Dundee, Scotland. All regular medications had to be stable for 6 weeks prior to entry into the study and during the study period. Patients were excluded if they had evidence of heart failure at screening or a previous history of heart failure. They were also excluded if they were on insulin or warfarin therapy. For comparison purposes, 9 age-matched healthy volunteers, recruited via advertisement, were also studied using the same protocol (below), but without Holter monitoring.
Study protocol
This study followed the protocol of two of our previous studies looking at the effects of spironolactone on endothelial function in patients with heart failure [20, 29]. One month of therapy with spironolactone (50 mg/day) was compared to placebo in a randomised, double-blind trial with a 2-week washout period between treatments. All patients attended at intervals of 1 to 2 weeks to allow monitoring of potassium levels and dose titration of spironolactone. Dose titration was performed by a research nurse, who was not involved in the acquisition or analysis of results. Each subject attended for endothelial function studies, 24-hour Holter monitoring and blood tests as described below at the end of each treatment phase.
All subjects gave written informed consent to participate in the study, which had been given prior approval by the Tayside Committee for Medical Research Ethics. The investigation conformed to the principles outlined in the Declaration of Helsinki.
Endothelial function studies
Endothelial function was studied by the technique of strain gauge plethysmography, which has been described in detail [30]. Studies were performed at the same time each day in each patient, after an overnight fast. Oral hypoglycaemic tablets were omitted on the morning of the study, all other therapies were taken as normal. Subjects were asked to refrain from caffeine or alcohol for at least 12 hours prior to each study visit. Endothelial function testing was performed with the patient supine, in a quiet, temperature-controlled room (20–24 °C). After the subject had rested for 15 min in a supine position, the brachial artery of the non-dominant arm was cannulated under local anaesthesia using a 27-gauge needle mounted on a 16-gauge epidural catheter. All drugs were infused using a constant rate infuser at a rate of 1 ml·min−1. After 15 minutes of saline infusion, baseline readings for forearm blood flow (FBF) were obtained. After 3 baseline readings, vasoactive substances as outlined below were infused, with a washout in between, to allow flow to return to baseline. FBF measurements were taken for the final 2 minutes of each 7-minute infusion. FBF was measured in the control arm and the infusion arm. Control arm blood flow was measured to confirm that the vasoactive compounds were not systemically active. Control arm blood flow can also be used to control for basal blood flow alterations during the experiment, and this is why the ratio between the infusion arm and the control arm was calculated.
Vasoactive substances
Acetylcholine (Novartis, Frimley, UK) was infused at doses of 50 and 100 nmol·min−1. This was followed by sodium nitroprusside (SNP) (Faulding Pharmaceuticals, Leamington Spa, UK) at a single dose of 37.8 nmol·min−1. Each substance was infused for 5 minutes before FBF measurements were taken. Acetylcholine is an endothelium-dependent and SNP an endothelium-independent vasodilator.
Heart rate variability
We analysed 24-hour ECG tracings using a Reynolds pathfinder 600 series workstation. Tapes were analysed in the time and frequency domains previously detailed in the literature [31]. In the time domain tracings were analysed for the standard deviations of R-R intervals within each 5-minute time period (SDNNI), the root mean square of differences of successive R-R intervals (RMSSD), the standard deviation of all R-R intervals (SDNN) and the standard deviation of mean R-R intervals every 5 min (SDANN). In the frequency domain tracings were analysed for low frequency, high frequency, the ratio between low-frequency and high-frequency elements and high-frequency and low-frequency elements normalised to heart rate. RMSSD and SDNNI reflect short-term heart rate variability, whereas SDNN and SDANN are attributed to longer term changes in heart rate variability. High-frequency power is thought to reflect parasympathetic status. The interpretation of low-frequency power is controversial, but the low frequency : high frequency ratio is thought to reflect sympathovagal imbalance.
Blood tests
Blood samples for screening of urate, fasting glucose, urea and electrolytes were analysed in the Department of Biochemical Medicine, Ninewells Hospital, Dundee, using a Roche 917 analyser. HbA1c was measured using a Menarini (HA-8140) analyser. Renin and aldosterone were analysed in duplicate by RIA kits (Diasorin, Wokingham, UK). Intra-assay coefficients of variation were 5.2% and 8.1% respectively for renin and aldosterone. For patients not on angiotensin II inhibition, angiotensin II was assayed by a RIA kit (Immuno-Diagnostics Systems, Bolon, UK). Intra-assay coefficients of variation were 5%. For patients on angiotensin II inhibition, angiotensin II was analysed using the assay of Morton and Webb [32], which is sensitive enough to detect low levels of angiotensin II even in the presence of high levels of angiotensin I. Plasma cortisol was measured using an RIA kit (Diasorin), the intra-assay coefficient of variation was 4.8%.
Statistical analyses
Power calculations were performed by Dr Simon Ogston, Medical Statistician, and were based on data from previous endothelial function studies in our department.
All statistical analyses were performed using SPSS version 10.1 for Windows after a treatment order effect had been excluded.
Results are analysed as change in parameter on active treatment compared to placebo. FBF (ml·min−1·100 ml−1 forearm volume) was analysed both in the infusion arm alone and as the ratio between the infusion arm and the control arm. Each was expressed as the percentage change in blood flow from the preceding baseline. The infusion arm alone was used for the primary analyses as there is evidence to suggest that for vasodilator substances this method of analysis is the most reproducible [33]. Data were also analysed for FBF in the infusion arm as a ratio of the control arm. Between-patient characteristics were analysed using independent samples t test. FBF and characteristics between treatments were analysed using two-way ANOVA. When more than three factors were considered for analysis at one time a Bonferroni adjustment was also applied. A p value of less than 0.05 was considered significant. Results are expressed in the text as means ± SEM.
Results
Subject characteristics
Baseline subject characteristics are detailed in Table 1.
The normal volunteers were age-matched to the diabetic group, but as would be expected they had significantly lower body weight (p=0.04), BMI (p=0.02) and HbA1c (p<0.001) than the diabetic subjects. There was no significant difference in blood pressure between the healthy volunteers and the diabetic subjects (p=0.36 and 0.94 for systolic and diastolic blood pressure respectively).
Of the Type 2 diabetic subjects, 20 were already taking ACE inhibitors and 22 were not on any ACE inhibition. Importantly, all 20 subjects on ACE inhibition met the entry criteria (a previous cardiovascular event or another cardiovascular risk factor) for the diabetic subpopulation in the HOPE study [15].
The differences between the patients on ACE inhibition and those who were not were largely non-significant apart from the fact that more patients in the ACE inhibition group had a history of hypertension (17 vs 3, p<0.001). However blood pressure was the same in each group at screening 143/75 mm Hg (22/11) in the ACE inhibitor group vs 146/81 mm Hg (14/7) in the group not on ACE inhibition (p=NS) (Table 2).
Treatment
The average dose of spironolactone was 47.5 mg/day; all but six patients managed to tolerate 50 mg/day after the initial dose titration. Increases in potassium levels limited full-dose spironolactone treatment in these six patients.
Values for haemodynamic and biochemical characteristics of the diabetic subjects and healthy volunteers for each study day are shown in Tables 3 and 4. Values for haemodynamic and biochemical characteristics of the diabetic subjects, grouped according to ACE inhibition therapy, are shown in Table 5.
Blood pressure
There were no significant differences in systolic or diastolic blood pressure between treatment phases when the Type 2 diabetic group was considered as a whole (128/72 mm Hg [20/9] during placebo and 128/71 mm Hg [27/9] during spironolactone). Similarly, blood pressure was not altered by spironolactone in the healthy volunteers (129/68 mm Hg [10/7] during placebo vs 135/75 mm Hg [8/5] during spironolactone). However when the diabetic group was split according to whether baseline therapy included ACE inhibition therapy, the 20 patients not on ACE inhibition had a significant fall in systolic blood pressure when on spironolactone relative to placebo (7.91±3.46 mm Hg, p=0.03).
Biochemistry
Spironolactone resulted in a significant decrease in sodium (p=0.05) and increases in renin (p=0.001), angiotensin II (p=0.003) and aldosterone (p=0.001) in the healthy volunteers, relative to placebo.
In the diabetic subjects there was a significant increase in potassium during spironolactone treatment (p<0.001). Uric acid increased by 0.03±0.01 mmol·l−1 (p<0.001) during spironolactone treatment and HbA1c increased by 0.24±0.08% (p=0.003). All of these biochemical changes remained significant irrespective of ACE inhibition therapy status (Table 5). Spironolactone treatment resulted in a highly significant increase in the levels of renin, aldosterone and angiotensin II; the percentage increase in these parameters was similar in those treated and those not treated with ACE inhibitors (Table 5). Spironolactone also significantly increased plasma cortisol (Table 3).
Endothelial function
Basal blood flow in both the infusion and the control arm did not differ between placebo and active treatments. There was no significant change in flow in the control arm during study days. In addition, control arm FBF at any point during endothelial function testing did not differ between active treatment and placebo.
All endothelial function results were similar whether analysed in the infusion arm alone or as the ratio between the infusion and control arm. Therefore results presented for endothelial function are those assessed in the infusion arm only.
When comparing the response of the healthy volunteers to acetylcholine during placebo with that of the diabetic patients, it was seen that the former responded to a much greater degree than diabetic subjects. Their response to acetylcholine was greater by 106.66±46.56% (95% CI 8.25–192.98%, p=0.03).
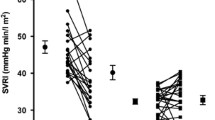
Spironolactone had no effect on FBF response to acetylcholine or SNP (p=0.5) in the healthy volunteers when compared to placebo. The mean difference in response to acetylcholine between spironolactone and placebo was −6.9±45.84% (95% CI −99.59 to 85.70%, p=0.88). In the Type 2 diabetic subjects, spironolactone treatment resulted in a significant worsening, relative to placebo, in the FBF response to acetylcholine of 44.56±14.56% (p=0.003), i.e. the FBF response to acetylcholine fell from 250% on placebo to 205% during spironolactone treatment (Fig. 1). The worsening of endothelial function on spironolactone compared to placebo occurred regardless of whether spironolactone was given first or not. This decline in endothelial function correlated significantly with the increase in HbA1c seen during spironolactone treatment (r=0.42, p=0.01).
Subgroup analysis of subjects on ACE inhibition (i.e. those subjects who fulfilled the entry criteria for the HOPE study) showed that the worsening of endothelial function still occurred in these patients and was highly significant. In patients on ACE inhibition, spironolactone resulted in a worsening, relative to placebo, in the FBF response to acetylcholine of 57.61±15.56% (p<0.001).
There was no significant difference in response to SNP between the active treatments and the placebo arm for all diabetic patients, the difference between spironolactone and placebo totalling 1.51±22.0% (p=0.95). Subgroup analysis of patients treated with ACE inhibition also failed to demonstrate a significant difference in response to SNP between spironolactone and placebo (p=0.79).
Heart rate variability
Heart rate variability was not assessed in the healthy volunteers. A total of 31 diabetic patients had data from 24-hour Holter monitoring that was of a sufficient quality to analyse. Results are given in Table 3.
Spironolactone significantly decreased RMSSD, a measure of short-term variability by 1.99±0.93 ms (p=0.03). SDNNI, another measure of short-term heart rate variability, was also decreased by spironolactone treatment, but this did not quite reach significance (difference between spironolactone and placebo: −2.55±1.47 ms, p=0.09). There was no significant effect of spironolactone on the longer term measures of heart rate variability, SDNN and SDANN. Although spironolactone did not have a significant effect on low-frequency and high-frequency power, when these were normalised, there was a significant increase in low-frequency and a significant decrease in high-frequency power on spironolactone compared to placebo (2.00±0.91 normalised units [nu], p=0.03, and −1.98±0.94 nu, p=0.04 respectively). Spironolactone treatment also resulted in an increase in the ratio of low-frequency to high-frequency power by 0.40±0.19 (p=0.04).
Discussion
Our principle finding is that spironolactone worsened already impaired endothelial function in these subjects with Type 2 diabetes. This worsening of endothelial function also occurred in the subgroup of diabetic patients who were already on ACE inhibition and who fulfilled entry criteria for the HOPE trial, i.e. patients who were known to benefit from ACE inhibition. This is in contrast to our previous studies in patients with heart failure [20, 29].
Endothelial function measured both in the coronary circulation and in the brachial artery has been shown in numerous studies to be a reliable predictor of future cardiovascular events [6, 7, 8, 9, 10, 11, 12]. Furthermore, most therapies that improve endothelial function usually lead to an improvement in prognosis [24, 25, 26]. Indeed, a recent editorial in Circulation called endothelial function a barometer of vascular health, representing an orchestrated response to all the processes that contribute to atherosclerosis development and progression [27]. It is therefore of concern that aldosterone blockade causes endothelial dysfunction in this patient group.
Spironolactone also increased angiotensin II, urate and HbA1c (and the degree of worsening of endothelial function during spironolactone treatment correlated significantly with the increase in HbA1c), making it unlikely that our findings on endothelial function were due to chance, as angiotensin II, urate and HbA1c are each known to be associated with endothelial dysfunction [16, 34]. Not only did spironolactone increase three stimuli, each known individually to be associated with endothelial dysfunction, but our finding that spironolactone worsened endothelial function is highly statistically significant (p=0.003). All of these factors greatly increase the credibility of our main finding that spironolactone worsens endothelial function.
Contrary to what might have been anticipated, spironolactone still had a deleterious effect on endothelial function in patients who were on ACE inhibitors. Patients in the ACE inhibition group fulfilled the criteria for the HOPE study and therefore are known to benefit from renin-angiotensin-system blockade. However, unlike in patients with heart failure, spironolactone did not enhance the beneficial effect of ACE inhibitors in these diabetic patients, appearing on the contrary to be positively counterproductive. As with all medical therapies, there is a risk : benefit ratio, and it may be that the increase in angiotensin II (which was higher in the ACE inhibitor group after spironolactone treatment) and the worsening glycaemic control (as demonstrated by an increase in HbA1c) outweighed the potential benefits of aldosterone blockade.
HbA1c is a marker of long-term (<3 months) glycaemic control. Our study period, however, was only 1 month. Our results for HbA1c should therefore be treated with caution, although, if anything, it is likely that our study underestimated the adverse effect of spironolactone on HbA1c. Moreover, although the change in HbA1c was small, it is highly statistically significant (p=0.001), and therefore impossible to dismiss. This change was also accompanied by a 0.4 mmol.l−1 increase in fasting glucose, although this latter result did not reach statistical significance.
Our observation that spironolactone increased plasma cortisol is intriguing. It might be because glucocorticoids bind to some extent to mineralocorticoid receptors and that this route of cortisol clearance becomes relatively unavailable when spironolactone is bound to this receptor. One possibility is that this increase in cortisol contributes to an anti-inflammatory effect of aldosterone blockade.
We also demonstrated a small, but significant worsening of heart rate variability parameters during spironolactone treatment. Although these small changes may not be of great clinical significance, the fact that heart rate variability also deteriorated with spironolactone treatment makes it unlikely that the worsening of endothelial function is due to chance, since heart rate variability has been shown to be closely associated with nitric oxide activity [35, 36].
As would be expected, the diabetic patients in this study were more obese than the control subjects. We cannot therefore be sure whether diabetes or obesity led to the adverse effects of spironolactone. As Type 2 diabetes and obesity are intimately associated, it would be difficult to tease out the culprit here.
In essence aldosterone blockade seems to produce a variety of different effects. These effects are favourable in heart failure, and also in heart failure patients with diabetes [18], but they appear to be unfavourable with respect to the prognostic markers of endothelial function and heart rate variability in Type 2 diabetic patients without heart failure (including those already known to benefit from blockade of the renin-angiotensin system). Interestingly, the beneficial effects seen with angiotensin II withdrawal in LIFE and HOPE were greater in the diabetic subgroups of those studies, but the opposite trend was seen with aldosterone blockade in EPHESUS, which again gives credence to our findings.
It therefore seems likely that the effects of spironolactone are disease-specific. So far we only know its vascular effects in heart failure and in Type 2 diabetes. In between these two extremes are various patient populations who still might obtain more benefit than harm from aldosterone blockade, e.g. patients with frank hypertension, or other vascular patients. It would therefore be worthwhile to study the effects of aldosterone blockade in these patient groups.
Although our findings only pertain to the particular subgroup of diabetic patients studied here, we recommend that caution be exercised when considering the prescription of aldosterone blockade to diabetic patients without heart failure.
Perspectives
Our study demonstrates a potential downside for aldosterone blockade and raises an important caveat for its future use. It is important to recognise and highlight this downside, so that aldosterone blockade, which is a novel therapy currently under intense investigation, can be allocated an appropriate place in the whole of cardiovascular therapy.
Abbreviations
- FBF:
-
forearm blood flow
- nu:
-
normalised units
- RMSSD:
-
root mean squared standard deviation
- SDANN:
-
standard deviation of the average R-R intervals over 5-minute periods
- SDNN:
-
standard deviation of R-R intervals
- SDNNI:
-
standard deviation of the R-R intervals within each 5-minute period
- SNP:
-
sodium nitroprusside
References
No authors listed (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853
McVeigh GE, Brennan GM, Johnston GD et al. (1992) Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 35:771–776
Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P (2001) Endothelial dysfunction and type 2 diabetes. Part 2: altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab 27:436–447
Liao D, Sloan RP, Cascio WE et al. (1998) Multiple metabolic syndrome is associated with lower heart rate variability. The Atherosclerosis Risk in Communities Study. Diabetes Care 21:2116–2122
Singh JP, Larson MG, O’Donnell CJ et al. (2000) Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol 86:309–312
Halcox JP, Schenke WH, Zalos G et al. (2002) Prognostic value of coronary vascular endothelial dysfunction. Circulation 106:653–658
Schachinger V, Britten MB, Zeiher AM (2000) Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101:1899–1906
Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R (2002) Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 40:505–510
Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A (2000) Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101:948–954
Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T (2001) Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104:2673–2678
Gokce N, Keaney JF Jr, Hunter LM et al. (2003) Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 41:1769–1775
Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA (2002) Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 105:1567–1572
Kontopoulos AG, Athyros VG, Didangelos TP et al. (1997) Effect of chronic quinapril administration on heart rate variability in patients with diabetic autonomic neuropathy. Diabetes Care 20:355–361
O’Driscoll G, Green D, Maiorana A, Stanton K, Colreavy F, Taylor R (1999) Improvement in endothelial function by angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 33:1506–1511
No authors listed (2000) Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 355:253–259
Struthers AD (1999) Why does spironolactone improve mortality over and above an ACE inhibitor in chronic heart failure? Br J Clin Pharmacol 47:479–482
Davies J, Struthers A (2002) The potential benefits of aldosterone antagonism in Type 2 diabetes mellitus. J Renin Angiotensin Aldosterone Syst 3:150–155
Pitt B, Zannad F, Remme WJ et al. (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341:709–717
Pitt B, Remme W, Zannad F et al. (2003) Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348:1309–1321
Farquharson CA, Struthers AD (2000) Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation 101:594–597
MacFadyen RJ, Barr CS, Struthers AD (1997) Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 35:30–34
PROGRESS Collaborative Group (2001) Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 358:1033–1041
Lindholm LH, Ibsen H, Dahlof B et al. (2002) Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359:1004–1010
O’Driscoll G, Green D, Rankin J, Stanton K, Taylor R (1997) Improvement in endothelial function by angiotensin converting enzyme inhibition in insulin-dependent diabetes mellitus. J Clin Invest 100:678–684
Husain S, Andrews NP, Mulcahy D, Panza JA, Quyyumi AA (1998) Aspirin improves endothelial dysfunction in atherosclerosis. Circulation 97:716–720
Mather KJ, Verma S, Anderson TJ (2001) Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol 37:1344–1350
Vita JA, Keaney JF Jr (2002) Endothelial function: a barometer for cardiovascular risk? Circulation 106:640–642
Tsuji H, Larson MG, Venditti FJ Jr et al. (1996) Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94:2850–2855
Macdonald JE, Kennedy N, Struthers AD (2004) Effects of spironolactone on endothelial function, vascular angiotension converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart 90:765–770
Benjamin N, Calver A, Collier J, Robinson B, Vallance P, Webb D (1995) Measuring forearm blood flow and interpreting the responses to drugs and mediators. Hypertension 25:918–923
No authors listed (1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93:1043–1065
Morton JJ, Webb DJ (1985) Measurement of plasma angiotensin II. Clin Sci (Lond) 68:483–484
Walker HA, Jackson G, Ritter JM, Chowienczyk PJ (2001) Assessment of forearm vasodilator responses to acetylcholine and albuterol by strain gauge plethysmography: reproducibility and influence of strain gauge placement. Br J Clin Pharmacol 51:225–229
Williams SB, Goldfine AB, Timimi FK et al. (1998) Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation 97:1695–1701
Chowdhary S, Vaile JC, Fletcher J, Ross HF, Coote JH, Townend JN (2000) Nitric oxide and cardiac autonomic control in humans. Hypertension 36:264–269
Spieker LE, Corti R, Binggeli C, Luscher TF, Noll G (2000) Baroreceptor dysfunction induced by nitric oxide synthase inhibition in humans. J Am Coll Cardiol 36:213–218
Acknowledgements
This research was funded by the British Heart Foundation. Lesley McFarlane and Valerie Godfrey contributed substantial laboratory-based time and effort during this study.
Statement on conflicts of interest
A. D. Struthers has received honoraria from Pfizer, who make the aldosterone receptor blocker, Eplerenone.
Author information
Authors and Affiliations
Corresponding author
Additional information
Statement on conflicts of interest: A.D. Struthers has received honoraria from Pfizer, who make the aldosterone receptor blocker, Eplerenone
Rights and permissions
About this article
Cite this article
Davies, J.I., Band, M., Morris, A. et al. Spironolactone impairs endothelial function and heart rate variability in patients with Type 2 diabetes. Diabetologia 47, 1687–1694 (2004). https://doi.org/10.1007/s00125-004-1510-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1510-8