Abstract
Aims/hypothesis
We tested whether chronic overstimulation by levels of hyperglycaemia commonly found in Type 2 diabetes can irreversibly desensitise beta cells and, if so, whether desensitisation relates to the reduction of insulin content and/or the number of beta cells.
Methods
We transplanted islets from Wistar-Furth rats under the kidney capsule to neonatally streptozotocinised recipients. Recipients received daily vehicle, diazoxide (100 mg/kg) or the selective activator of beta cell type K+-ATP channels 6-chloro -3-(1-methylcyclopropyl) amino-4H-thienol [3,2-e]-1,2,4-thiadiazine 1,1-dioxide (NN414) (3 mg/kg) intragastrically for at least 9 weeks. Endpoint measurements were made exactly 7 days after cessation of treatment.
Results
Blood glucose did not differ between groups (mean of total: 13.2±1.4 mmol/l). C-peptide levels were significantly depressed in drug- versus vehicle-treated rats 3 to 4 hours after the last gastric tubing event, but not at endpoint. Insulin responses to 27 mmol/l glucose from perifused grafts were not significant after vehicle (median increment 18×10−3 µU·islet−1·min−1) but were significant per se and versus vehicle in the diazoxide and NN414 groups (median 107 and 83×10−3 respectively). Rising second-phase secretion was seen only in the drug-treated groups. Stimulation by 25 mmol/l KCl, together with 0.5 mmol/l 3-isobutyl-1-methylxanthine and 3.3 mmol/l glucose, was enhanced in the drug-treated groups (p<0.05 versus vehicle). Graft insulin content did not differ between groups, nor did percentage of beta cells (between 67 and 68% of endocrine cells).
Conclusions/interpretation
Chronic overstimulation by moderate hyperglycaemia damages signalling events including those required for glucose-induced insulin secretion. This signal transduction defect occurs in the absence of any effect on islet macro-morphometry or insulin stores.
Similar content being viewed by others
Introduction
Prolonged hyperglycaemia is known to negatively affect beta cell function. Leahy et al. demonstrated in normal rats that 48 hours of hyperglycaemia desensitised the pancreas to subsequent in vitro stimulation with glucose, i.e. the insulin response to glucose was abolished [1]. Sako and Grill then showed that the desensitisation by hyperglycaemia could be completely prevented if rats were co-infused with diazoxide [2]. Diazoxide inhibits glucose-induced insulin secretion through activation of ATP-sensitive potassium (K+-ATP) channels in the cell membrane of beta cells [3]. Diazoxide also protected against desensitisation in isolated islets from rat [4] and human [5, 6] pancreases. Therefore, it was concluded that overstimulation by elevated glucose was responsible for the desensitisation observed.
These and other reports have led to the distinction of different effects of hyperglycaemia on the beta cell [7]. The term “glucotoxicity” stands for negative effects exerted directly by glucose and/or its metabolites. Overstimulation (or “beta cell exhaustion”) stands for an indirect effect of long-term elevated glucose. (However, other conditions could also lead to overstimulation).
Following a 48-hour period of hyperglycaemia, insulin secretion swiftly (within 24 h) normalised when blood glucose was normalised [1]. The 48-hour time frame of hyperglycaemia is different from the chronic hyperglycaemia present in diabetes. Therefore these previous studies do not exclude the possibility of irreversible effects from overstimulation in diabetes and it seems clinically important to decide whether desensitisation after a prolonged period of hyperglycaemia is reversible or not. To answer this question, transplantation studies, although technically difficult and labour-intensive, are needed. To test “proof of principle”, we transplanted islets to rats that had been made severely diabetic by streptozotocin at adulthood (blood glucose >22 mmol/l) [8]. Eight weeks of diazoxide treatment followed by 1 week of no treatment improved insulin secretion in response to arginine but not to glucose, and in parallel increased insulin content and preproinsulin of the grafts. These experiments strongly indicated that diazoxide can protect against irreversible damage to beta cells.
However, extreme hyperglycaemia may induce other metabolic abnormalities and is not usually seen in subjects with Type 2 diabetes. Therefore, it was necessary to test effects of overstimulation at levels of hyperglycaemia closer to the clinical situation than those used in the previous study. We therefore transplanted islets to neonatally streptozotocin-diabetic rats, which typically develop only moderate hyperglycaemia [9]. To ascertain the validity of data obtained with diazoxide, we compared them with those of a more potent and selective activator of the ATP-sensitive potassium channels of the beta cells, namely the new compound 6-chloro -3-(1-methylcyclopropyl) amino-4H-thienol [3,2-e]-1,2,4-thiadiazine 1,1-dioxide (NN414 ) [10]. Such testing was also important for therapeutic considerations, since side effects of diazoxide limit its use in clinical trials [11, 12].
In a three-arm study the transplant-bearing rats received vehicle, diazoxide or NN414 for at least 9 weeks. We stopped all treatment 7 days before assembling ex vivo biochemical and histo-pathological data on graft function and survival.
Materials and methods
Animals
Female and male Wistar-Furth rats were obtained from Scanbur (Sollentuna, Sweden). Female rats served as donors and male syngeneic rats as recipients in the transplantation experiments. Male rats were made diabetic by an intravenous injection with 90 mg/kg streptozotocin (Sigma, St. Louis, Mo., USA) at day 1 after birth. Moderate diabetes was induced and confirmed at day 3 by blood glucose levels above 10.0 mmol/l. The mean blood glucose level at transplantation was 17.8±1.1 mmol/l and was essentially the same in all three groups (Table 1). Also body weight of the diabetic rats at transplantation was similar between groups (Table 1).
Isolation and transplantation of islets
Islets of Langerhans were isolated by collagenase treatment [13] from 12 to 15-week-old female syngeneic rats. Islets were cultured overnight in RPMI 1640 (SVA, Uppsala, Sweden). Then, two islet grafts with 150–220 and 20–50 islets respectively were transplanted under the left kidney capsule as previously described [8]. To minimise inter-individual variations between donors, islets isolated from two to three donor rats were mixed and then divided into three equal portions, one-third being transplanted to a rat belonging to the vehicle-treated, one-third to the diazoxide-treated and one-third to the NN414-treated group. The number of islets transplanted to each rat was purposely kept low enough not to reverse diabetes in the recipient rats.
Experimental protocols
The Stockholm Ethics Committee for Animal Experiments approved the experimental protocols. Following transplantation, the recipient rats were administered vehicle, diazoxide (100 mg/kg) or NN414 (3 mg/kg) once daily between 08.00 and 10.00 hours. Diazoxide and NN414 were dissolved in 2% NaOH. The vehicle component consisted of glycerol 5 vol %, gelatine 0.5%, 40 vol % and carboxy-methyl-cellulose 2%, 55 vol %. The treatment lasted for 77±2 (range: 63–87) days with no significant differences between the groups. The treatment period was followed by exactly 7 days of no treatment in every animal. The animals had free access to water and the standard laboratory chow for rats (Scanbur, Stockholm, Sweden) during the experimental period.
Non-fasting blood glucose levels were measured once every week between 09.00 and 11.00 hours, just before medication throughout the experimental period. Levels of plasma C-peptide were measured at the end of the treatment period (3–4 hours after the last gastric tubing event) and at the end of the study.
Perifusion of kidney grafts
The perifusion technique was adapted from that used in mouse transplantation experiments [14]. Graft-bearing kidneys were isolated and the grafts carefully excised. Grafts were then perifused as previously described [4] with Krebs-Ringer bicarbonate buffer supplemented with 0.2% of bovine serum albumin (Sigma), and with 3.3 mmol/l glucose. The flow rate was kept at 0.2 ml/min by a peristaltic pump. After a 30-min equilibration period, each graft was stimulated for 30 min with 27 mmol/l glucose. This stimulation was followed by an equivalent period of perifusion with 3.3 mmol/l glucose. Finally, grafts were stimulated with a combination of 25 mmol/l KCl and 0.5 mmol/l 3-isobuthyl-1-methylxanthine (IBMX) on a background of 3.3 mmol/l of glucose. Effluents were collected every 1 to 2 min and stored at −20 °C until insulin assay. Following perifusions, the larger grafts were processed for structural analysis and the smaller grafts primarily for insulin contents as outlined below.
Assays
Levels of blood glucose were assayed by the glucose oxidase method (Accutrend Sensor Glucose, Boehringer Mannheim, Mannheim, Germany). Plasma levels of C-peptide were assayed by radioimmunoassay (Linco Research, St. Charles, Mo., USA). The insulin content of the smaller grafts (containing 20–50 islets) was extracted as previously reported [15]. Immunoreactive insulin in effluents and extracts was measured by radioimmunoassay [16].
Histopathological and morphometrical analysis
The islet transplants, which had been identified by naked-eye inspection and excised together with a brim of the adjacent renal cortex, were conventionally fixed by immersion in 10% neutral formalin, dehydrated and embedded in paraffin. For orientation purposes, sections about 4 µm thick were cut and stained with haematoxylin and eosin. After ascertaining that a graft had been hit by the microtome, a whole series of five to ten additional, consecutive, 4-µm thick sections were cut and used for immunohistochemical (IHC) analyses of the presence of the four major islet hormones in the parenchymal cells of the grafts. At the end of such a series, an additional section was cut, haematoxylin- and eosin-stained, and compared with the first section. By checking whether or not the area of the graft in the cut sections increased or decreased in size, we gained an indication as to whether or not the cut sections were from the central parts of the graft. If not, additional consecutive sections were cut and used for the IHC analyses. By these means, an estimate was made of the size and shape of the graft.
Conventional peroxidase-anti-peroxidase IHC investigations were made using Dako equipment (Dako, Glostrup, Denmark). Thus, the sections were immunostained on the Tech-Mate-500-Plus Autostainer, using the Chem-Mate Detection Kit. The primary antibodies were all polyclonal. For the detection of insulin cells, Dako guinea-pig anti-porcine insulin A564 antiserum was used at a dilution of 1:1200. The somatostatin cells were identified by means of their rabbit anti-human somatostatin AO566 antiserum, using a working dilution of 1:12 000. For assessing glucagon cells, rabbit anti-glucagon A565 antiserum was applied at a working dilution of 1:500. Lastly, for the detection of the pancreatic polypeptide (PP) cells, rabbit anti-human PP AO619 antiserum was used at a working dilution of 1:50 000. The negative controls were those recommended by Dako.
In the cut sections, only cells displaying an intact nucleus and a distinct immunoreactivity of granular type in the cytoplasm were counted. Each counting was repeated at least twice in order to check its accuracy. There were no major difficulties in distinguishing the islet parenchymal cells from blood vessel endothelium, connective tissue fibroblasts/fibrocytes or the epithelial cells of the adjacent renal tubuli. When the total number of immunoreactive islet parenchymal cells had been obtained, we checked whether that number was of the same magnitude as that obtained when the total number of islet parenchymal cells in the grafts had been assessed in the haematoxylin- and eosin-stained sections, both in the first and last ones of the series of consecutive sections. By this means, we gained an indication as to whether or not any degranulated or otherwise non-immunoreactive parenchymal cells were present in the islet grafts.
The haematoxylin- and eosin-stained sections, as well as the immunostained ones, were assessed blindly, i.e. without information about which experimental group they originated from. Before the statistical calculations started, the numerical values of the morphometric data were graphically checked for the presence of a Gaussian (“normal”) distribution.
Statistical analysis
Data in tables and figures are expressed as means ± SE. For multiple comparisons, one-way repeated measures ANOVA was performed. For comparisons between two means, Student’s t test (two-tailed) for paired observations was performed. A p value of less than 0.05 was considered significant.
Results
In vivo measurements
Body weights and blood glucose
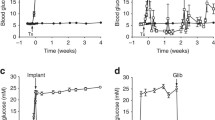
Body weight of the rats did not change significantly in any group during the experimental period (Table 1, Fig. 1a). During the first week, blood glucose was non-significantly and temporarily higher in the diazoxide- and NN414-treated group than in vehicle-treated animals (p=0.059). The degree of hyperglycaemia diminished in all groups during the treatment period and at death (Table 1). The mean blood glucose of the animals in the study was thus much lower than before treatment (17.8±1.1 vs 13.2±1.4 mmol/l). Most of the reduction of blood glucose occurred early during treatment (Fig. 1b). At the end of treatment there were no significant differences in levels of blood glucose between the groups.
Plasma C-peptide
Plasma C-peptide was measured 3 to 4 hours after the last gastric tubing event. Levels were then significantly lower in diazoxide- and NN414-treated rats than in vehicle-treated rats (Table 1). Plasma C-peptide was measured again at the time of death, i.e. on the seventh day after treatment ended. At that time point levels were similar in all three groups.
In vitro measurement
Insulin release during perifusions
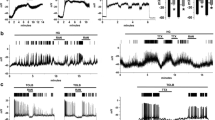
The mean incremental insulin response to 27 mmol/l glucose was small and non-significant in grafts to vehicle-treated diabetic rats (0.04±0.02 µU·islet−1·min−1, mean ± SE) (Figs. 2, 3). The median increment during stimulation by glucose was only 18×10−3 µU·islet−1·min−1. There was no tendency for insulin secretion to increase during the stimulation period (Fig. 2). In contrast, the insulin response to glucose was significant in grafts to the diazoxide-treated rats (0.13±0.04 µU·islet−1·min−1, mean ± SE). The median increment during stimulation by glucose was 107×10−3 µU·islet−1·min−1. There was a successive rise in secretion over time. Also grafts to NN414-treated rats responded well to glucose (0.15±0.06 µU·islet−1·min−1, mean ± SE). The median increment during stimulation by glucose was 83×10−3 µU·islet−1·min−1 from grafts to NN414-treated rats. The kinetics of secretion were similar to those of diazoxide.
Incremental (= basal secretion at 3.3 mmol/l glucose subtracted) insulin responses to 27 mmol/l glucose as well as to 25 mmol/l KCl, 0.5 mmol/l IBMX and 3.3 mmol/l glucose. Results are from perifusion of transplants containing 150–220 islets. (○) vehicle-, (●) diazoxide- and (□) NN414-treated, n=8 in all groups. The triangle shows data from stimulation with 27 mmol/l glucose of transplants from non-diabetic rats obtained in a separate series of experiments, n=4. Some error bars were omitted for clarity. G, glucose; IBMX, 3-isobutyl-1-methylxanthine
Incremental insulin release in response to 27 mmol/l glucose (22–80 min) in perifusions of transplants (see Fig. 2) and to 25 mmol/l KCl, 0.5 mmol/l IBMX and 3.3 mmol/l glucose (81–112 min). Values are calculated as area under the curve and expressed as per islet per min. Bars refer to graft secretion from vehicle- (white), diazoxide- (black) and NN414-treated (grey) animals
To broadly assess the extent to which diazoxide and NN414 treatment restored glucose-induced insulin secretion to normal, we included (Fig. 2) data from perifused grafts to non-diabetic recipients. These data were obtained during the same time period as the present three-arm study, but in a separate series of experiments. The kinetics of insulin release of grafts from untreated non-diabetic rats was quite similar to that of grafts from the drug-treated groups of the present study.
Combined stimulation with KCl and the phosphodiesterase inhibitor IBMX on a background of 3.3 mmol/l glucose elicited sizable insulin release from grafts both to the drug- and to the vehicle-treated groups of diabetic recipients (Figs. 2, 3). However, this response was more marked in grafts from diazoxide- or NN414-treated rats (0.55±0.16 µU·islet−1·min−1, mean ± SE and 0.56±0.16 µU·islet−1·min−1, mean ± SE respectively) than in grafts from the vehicle-treated ones (0.27±0.11 µU·islet−1·min−1, mean ± SE) (Fig. 3).
In order to validate the technique of perifusion of islet grafts, we compared responses from the large grafts containing at transplantation 150–220 islets with responses from the small grafts (20–50 islets) to the same animal. As expected, the release from the larger number of islets was less variable than that from the smaller grafts (results not shown); it was therefore natural to present data from the larger grafts. There was, however, a positive correlation between the response of the small and large transplant in an individual animal, both with regard to stimulation with glucose (r=0.5; p=0.03) and with regard to combined stimulation with KCl and IBMX (r=0.6; p=0.01).
Insulin content
The insulin content of the grafts did not differ significantly between treatment groups. The insulin content was 401±163 µU/islet in grafts from vehicle-treated, 490±179 µU/islet from diazoxide-treated and 318±108 µU/islet from NN414-treated rats.
In all groups, there was a negative correlation at death between insulin content of grafts and blood glucose (r=−0.64, p=0.006 for the three groups combined). In contrast, a positive correlation between insulin content and glucose-induced insulin release during perifusion was found only for vehicle-treated rats (r=0.72, p=0.046). For the diazoxide and NN414 groups the non-significant r values were −0.51 and 0.08 respectively.
Estimated fractional release
We used insulin content data from individual experiments to express insulin release in terms of fractional release. Strictly speaking, this was an estimation since insulin content was measured in the smaller graft to the kidney of each recipient, whereas insulin secretion was routinely measured in the larger graft. Furthermore, insulin content data were lacking in three of the 24 experiments due to technical reasons. The estimated incremental fractional insulin release showed a profile very similar to that of incremental insulin release (compare Figs. 2 and 4). The mean incremental fractional insulin response to 27 mmol/l glucose was 0.01±0.003, 0.07±0.03 and 0.06±0.02% per min for vehicle-, diazoxide- and NN414-treated animals respectively (p<0.03 for vehicle vs diazoxide and NN414 pooled together). Stimulation with KCl and IBMX on a background of 3.3 mmol/l glucose resulted in a fractional secretion of 0.08±0.02, 0.31±0.2 and 0.27±0.09% per min for vehicle, diazoxide and NN414, p=0.2.
Estimated incremental insulin fractional release (i.e. insulin release from large grafts divided by insulin content from small grafts). Conditions as in Fig. 2. G, glucose; IBMX, 3-isobutyl-1-methylxanthine
Histopathological, IHC and morphometrical observations
Apart from the presence of small islet haemorrhages [17] in a few islet grafts belonging to all three experimental groups, no light-microscopical structural lesions were found in the various kinds of islet parenchymal cells. The incidence of non-immunoreactive islet parenchymal cells was close to 0% (Table 2). Thus, degranulated islet cells or immature agranular ones [17] did not occur in the islet grafts. The total number of islet parenchymal cells in the kidney grafts varied rather widely. There was a positive correlation between the amount of transplanted islets and the amount of endocrine cells counted per section (r=0.397, p=0.0329, when all three treatment groups were included).
The percentage of beta cells was not clearly reduced in comparison with that from pancreatic islets of normal rats [17]. Similar findings were obtained with regard to glucagon and somatostatin immunoreactive cells [17]. Practically no PP cells were found in the islet grafts. This observation indicates that the origin of the islet transplants was from the corpus and/or cauda regions of the donor pancreatic glands and not from the dorsal lobes of the caput region [17]. Most importantly, the occurrence of insulin immunoreactive cells and other cell types in the transplants was quite similar between groups (Table 2).
Discussion
Three main findings, to our knowledge all novel, emerge from this transplantation study. First, long-term treatment with diazoxide during moderate hyperglycaemia produces lasting beneficial effects on insulin secretion. Second, these effects are not explained by effects on insulin content or the number of beta cells in the transplants. Third, the selective activator of beta cell ATP-sensitive potassium channels, NN414, reproduces the beneficial effects of diazoxide.
At first glance, it is surprising that blood glucose was affected only during the first week of treatment since an insulin inhibitory effect of diazoxide and NN414 (demonstrated here by lowered C-peptide levels) would be expected to raise blood glucose. However, diazoxide has insulin-sensitising effects in rodents [18, 19, 20] and in humans [12, 21]. There is also evidence that NN414 has insulin-sensitising effects [22]. At any rate, the comparable level of hyperglycaemia during the different treatments greatly facilitates the interpretation of our results.
Our findings indicate that diazoxide and NN414 preserved several aspects of normal beta cell responsiveness to glucose in the islet transplants. In particular, the time kinetics was very similar to that obtained in transplants to non-diabetic rats. However, we cannot exclude quantitative differences in the glucose response from grafts to drug-treated diabetic versus non-diabetic rats.
Also the response to non-nutrient secretagogues was enhanced by the long-term presence of diazoxide or NN414. This could indicate that non-glucose-dependent events of signal transduction are also permanently influenced by overstimulation. Alternatively, the influence of the simultaneous low glucose concentration (3.3 mmol/l) was enhanced.
Other investigators have proposed that the beneficial effects of diazoxide on insulin secretion are wholly secondary to the preservation of insulin stores [23]. As discussed elsewhere [7], there is evidence that this does not apply to all experimental conditions manifesting desensitisation by overstimulation. The present findings offer a particularly striking case in point.
The discrepancy between effects on insulin secretion and insulin stores is potentially important from a clinical perspective, as it resembles the situation in Type 2 diabetes, where profound desensitisation of insulin secretion to glucose occurs despite only a moderate reduction in insulin stores and beta cell volumes [24].
The lack of association between the beneficial effects on insulin secretion and the number of beta cells and insulin content differs from our findings in a previous transplantation study [8]. There, diazoxide treatment enhanced arginine-induced insulin secretion in parallel with an increase in islet insulin content and preproinsulin mRNA in the transplants. It is possible that the severe hyperglycaemia of the previous study gave rise to more intense overstimulation than the more moderate hyperglycaemia in the present study. Indeed, the level of chronic hyperglycaemia correlates with negative effects on insulin biosynthesis in an animal model of Type 2 diabetes [25], although the effects of overstimulation were not, as here, evaluated separately from the glucotoxic effects of hyperglycaemia.
A third important finding of our study was the similarity of beneficial effects exerted by the two different activators of K+-ATP channels. With the more beta-cell-selective compound NN414 we obtained essentially the same results as with the non-selective compound diazoxide. It is noteworthy that the same results were obtained with vastly different doses: 3 mg/kg NN414 and 100 mg/kg of diazoxide. This difference in dosage is in line with the much greater potency of NN414 to acutely inhibit glucose-induced insulin secretion [10].
Which mechanisms are behind the beneficial effects of the activators of K+-ATP channels? Any non-specific effect on glucose levels and/or food intake seems to be excluded by glucose levels and body weight patterns evolving in parallel to those of vehicle-treated rats. In vitro studies [6, 26] have demonstrated that overstimulation induces a rise in cytoplasmic Ca2+ [6], which could be damaging. However, such a Ca2+ abnormality would, according to current knowledge, give rise to apoptosis and a diminished number of beta cells, a situation not encountered here. Overstimulation with accelerated protein synthesis could give rise to endoplasmatic reticulum stress [27]. However, such an effect would lead to accelerated beta cell death, again not conforming with our data. On the other hand, our data are consistent with damage to mitochondria, since beta cells with damaged mitochondrial function retain normal insulin content [28]. Further studies are needed to test this notion.
In summary, this in vivo and ex vivo study has demonstrated that 9 weeks of treatment with two different openers of K+-ATP channels followed by 7 days of no treatment preserves glucose and non-nutrient regulation of insulin secretion from transplants to diabetic rats with moderate hyperglycaemia. Beneficial effects on insulin secretion are not associated with cellular insulin and the number of beta cells in the transplants.
Abbreviations
- IBMX:
-
3-isobutyl-1-methylxanthine
- IHC:
-
immunohistochemical
- K+-ATP channels:
-
ATP-sensitive potassium channels
- NN414:
-
6-chloro -3-(1-methylcyclopropyl) amino-4H-thienol [3,2-e]-1,2,4-thiadiazine 1,1-dioxide
- PP cells:
-
pancreatic polypeptide cells
References
Leahy J, Cooper H, Deal D, Weir G (1986) Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest 77:908–915
Sako Y, Grill V (1990) Coupling of B-cell desensitization by hyperglycemia to excessive stimulation and circulating insulin in glucose-infused rats. Diabetes 30:1580–1583
Trube G, Rorsman P, Ohno-Shosaku T (1986) Opposite effects of tolbutamide and diazoxide on the ATP-dependent potassium channel in mouse pancreatic B-cells. Pflugers Arch 407:493–499
Björklund A, Grill V (1993) B-cell insensitivity in vitro: reversal by diazoxide entails more than one event in stimulus-secretion coupling. Endocrinology 132:1319–1328
Björklund A, Grill V (1999) Enhancing effects of long-term elevated glucose and palmitate on stored and secreted proinsulin-to-insulin ratios in human pancreatic islets. Diabetes 48:1409–1415
Björklund A, Lansner A, Grill V (2000) Glucose-induced Ca2+ abnormalities in human pancreatic islets: important role of overstimulation. Diabetes 49:1840–1848
Grill V, Björklund A (2001) Overstimulation and beta cell function. Diabetes 50 [Suppl 1]:S122–S124
Hiramatsu S, Höög A, Möller C, Grill V (2000) Treatment with diazoxide causes prolonged improvement of beta-cell function in rat islets transplanted to a diabetic environment. Metabolism 49:657–661
Bonner-Weir S, Trent D, Honey R, Weir G (1981) Responses of neonatal islets to streptozotocin: limited B-cell regeneration and hyperglycemia. Diabetes 30:64–69
Nielsen FE, Bodvarsdottir T, Worsaae A et al. (2002) 6-Chloro-3-alkylamino-4H-thieno[3,2-e]-1,2,4-thiadiazine1,1-dioxide derivatives potently and selectively activate ATP-sensitive potassium channels of pancreatic beta-cells. J Med Chem 12:4171–4187
Greenwood R, Mahler F, Hales C (1976) Improvement of insulin secretion in diabetes after diazoxide. Lancet 1:444–447
Björk E, Berne C, Kämpe O, Wibell L, Oskarsson P, Karlsson A (1996) Diazoxide treatment at onset preserves residual insulin secretion in adults with autoimmune diabetes. Diabetes 45:1427–1430
Lacy PE, Kostianovsky M (1967) Method for the isolation of intact islets of Langerhans from the pancreas. Diabetes 16:35–39
Shi CL, Täljedal I-B, Rooth P (1993) The protective effect of verapamil on mouse islets transplanted under kidney capsule. Diabetes 38:510–515
Grill V, Efendic S (1983) Loss of a priming effect of glucose on A- and D-cell secretion in perfused pancreas from alloxan diabetic rats; role of insulin and alloxan. Diabetologia 24:47–51
Herbert V, Lau KS, Gotttlieb CW, Bleicher SJ (1965) Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab 25:1375–1384
Falkmer S, El-Salhy M, Titlbach M (1984) Evolution of the neuroendocrine system in vertebrates. A review with particular reference to the phylogeny and postnatal maturation of the islet parenchyma. In: Falkmer S, Håkanson R, Sundler F (eds) Evolution and tumour pathology of the neuroendocrine system. Elsevier, Amsterdam, pp 59–87
Alemzadeh R, Slonim AE, Zdanowicz MM, Maturo J (1993) Modification of insulin resistance by diazoxide in obese Zucker rats. Endocrinology 133:705–712
Aizawa T, Taguchi N, Sato Y et al. (1995) A study in a rat model of naturally occurring obese diabetes. J Pharmacol Exp Ther 275:194–199
Surwit RS, Dixon TM, Petro AE, Daniel KW, Collins S (2000) Diazoxide restores beta 3-adrenergic receptor function in diet-induced obesity and diabetes. Endocrinology 141:3630–3637
Qvigstad E, Kollind M, Grill V (2004) Nine weeks of bedtime diazoxide is well tolerated and improves beta-cell function in subjects with Type 2 diabetes. Diabet Med 21:73–76
Carr RD, Brand C, Bodvarsdottir TB, Hansen JB, Sturis J (2003) NN414, a SUR1/Kir6.2-selective potassium channel opener, reduces blood glucose and improves glucose tolerance in the VDF Zucker rat. Diabetes 52:2513–2518
Leahy J (1996) Beta-cell dysfunction with chronic hyperglycemia: “overworked beta cell” hypothesis. Diabetes Rev 4:298–319
Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU (1985) Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Path Res 4:110–125
Jonas J-C, Sharma A, Hasenkamp W et al. (1999) Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem 274:14112–14121
Iwashima Y, Abiko A, Ushikubi F et al. (2001) Downregulation of the voltage-dependent calcium channel (VDCC) beta-subunit mRNAs in pancreatic islets of type 2 diabetic rats. Biochem Biophys Res Commun 280:923–932
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110:1389–1398
Kennedy ED, Maechler P, Wollheim CB (1998) Effects of depletion of mitochondrial DNA in metabolism secretion coupling in INS-1 cells. Diabetes 47:374–380
Acknowledgements
This work was supported by NovoNordisk, Denmark; the Cancer Research Fund of St. Olav’s University Hospital (grant no. 80071), Trondheim, Norway; the Swedish Diabetes Association; the Swedish Medical Research Council (grant nos. 72X-14070 and 2003-6289); the Swedish Society of Medicine; the Fredrik and Ingrid Thuring Foundation and the Loo and Hans Osterman Foundation.
Potential conflict of interest: John Bondo Hansen is employed by and holds stock in Novo-Nordisk A/S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Björklund, A., Bondo Hansen, J., Falkmer, S. et al. Openers of ATP-dependent K+-channels protect against a signal-transduction-linked and not freely reversible defect of insulin secretion in a rat islet transplantation model of Type 2 diabetes. Diabetologia 47, 885–891 (2004). https://doi.org/10.1007/s00125-004-1386-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-004-1386-7