Abstract
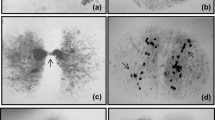
Mitotic anaphase cells of highly friable and embryogenic calluses which had been induced from immature embryos of two inbred lines of maize that have contrasting levels of heterochromatic knobs were analysed for the presence of abnormalities 3, 6, 9 and 12 months after the initiation of culture. A total of 500 typical anaphases was scored at each time point, and various aberrations, such as delay in the separation of sister chromatides, chromosome bridges (single, double and multiple) and chromosome fragments, were revealed to occur extensively in the cultures of both genotypes. Preparations after C-banding revealed that primary breakages often occurred inside knobs or at junction regions between the euchromatin and the heterochromatin of the knobs. Figures characterized by the delayed separation of sister chromatids, which originated preferentially at the knob level and was considered to be an initial event in the development of breakages, were observed at constant frequencies throughout the experiment. Increasing numbers of aberrant cells were detected with time, mainly due to the accumulation of cells with chromosome bridges and fragments. Several mitotic figures suggested the occurrence of breakagefusion-bridge cycles that were initiated by broken chromosomes. The overall frequencies of aberrant cells were similar for both genotypes, despite the differences in knob composition. However, callus cultures induced from the genotype having the higher level of knobs had more aberrant cells with abnormalities that involved several chromosomes, such as multiple bridges and multiple fragments.
Similar content being viewed by others
References
Aguiar-Perecin MLR (1985) C-Banding in maize. I. Band patterns. Caryologia 38:23–30
Aguiar-Perecin MLR, Fluminhan A (1992) Mitotic instability in callus cultures of inbred lines adapted to tropical regions. Maize Genet Coop Newsl 66:87–88
Armstrong CL, Phillips RL (1988) Genetic and cytogenetic variation in plants regenerated from organogenic and friable, embryogenic tissue cultures of maize. Crop Sci 28:363–369
Balzan (1978) Karyotype instability in tissue cultures derived from the mesocotyl of Zea mays seedlings. Caryologia 31:75–87
Benzion G, Phillips RL (1988) Cytogenetic stability of maize tissue cultures: a cell line pedigree analysis. Genome 30:318–325
Döbel P, Schubert I, Rieger R (1978) Distribution of heterochromatin in a reconstructed karyotype of Vicia faba as identified by banding and DNA-late replication patterns. Chromosoma 69:193–209
Edallo S, Zucchinali C, Perenzin M, Salamini F (1981) Chromosomal variation and frequency of spontaneous mutation associated with in vitro culture and plant regeneration in maize. Maydica 26: 39–56
Fluminhan A (1992) In vitro culture of maize (Zea mays L.) and analysis of its mitotic instability. MSc thesis, University of São Paulo, Piracicaba SP, Brazil (in Portuguese with abstract in English)
Fluminhan A, Aguiar-Perecin MLR, Santos JA (1996) Evidence for heterochromatin involvement in chromosome breakage in maize callus culture. Annals Bot (in press)
Green CE, Phillips RL (1975) Plant regeneration from tissue cultures of maize. Crop Sci 15:417–421
Hang A, Tsuchiya T, Stanwood PC, Roos EE (1994) Mitotic analysis of root tips from cryopreserved and artificially aged seeds of wheat. Cytologia 59:125–133
Hartwell LH, Smith D (1985) Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisae. Genetics 110:381–395
Hatch FT, Mazrimas JA (1977) Satellite DNA and cytogenetic evolution. In: Sparkes RS, Comings DE, Fox CF (eds) Molecular human cytogenetics (ICN-UCLA Symposium 7) Academic Press. London New York, pp 395–414
Holm C, Stearns T, Botsein D (1989) DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol Cell Biol 9:159–168
John B (1988) The biology of heterochromatin. In: Verma RS (ed) Heterochromatin: molecular and structural aspects. Cambridge University Press, Cambridge New York Melbourne Sydney, pp 1–147
Johnson SS, Phillips RL, Rines HW (1987) Possible role of heterochromatin in chromosome breakage induced by tissue culture in oats (Avena sativa L.). Genome 29:439–446
Kaeppler SM, Phillips RL (1993) DNA methylation and tissue culture-induced variation in plants. In Vitro Cell Dev Biol 29P: 125–130
Kaeppler SM, Phillips RL (1993) Tissue culture-induced DNA methylation variation in maize. Proc Natl Acad Sci USA 90: 8773–8776
Karp A (1991) On the current understanding of somaclonal variation. In: Milfin BJ (ed) Oxford surveys on plant molecular cell biology vol. 7. Oxford University Press, London, pp 1–58
Larkin PJ (1987) Somaclonal variation: history, method, and meaning. Iowa State J Res 61:393–434
Lee M, Phillips RL (1987) Genomic rearrangements in maize induced by tissue culture. Genome 29:122–128
Lee M, Phillips RL (1988) The chromosomal basis of somaclonal variation. Ann Rev Plant Physiol Plant Mol Biol 39:413–437
McClintock B (1939) The behavior in successive nuclear divisions of a chromosome broken at meiosis. Proc Natl Sci USA 25:405–416
McClintock B (1941) The stability of broken ends of chromosomes in Zea mays. Genetics 26:234–282
McCoy TJ, Phillips RL (1982) Chromosome stability in maize (Zea mays) tissue cultures and sectoring in some regenerated plants. Can J Genet Cytol 24:559–565
McCoy TJ, Phillips RL, Rines HW (1982) Cytogenetic analysis of plants regenerated from oat (Avena sativa) tissue cultures. High frequency of partial chromosome loss. Can J Genet Cytol 24: 37–50
Mohanty BD, Paul NK, Ghosh PD (1986) Chromosomal behaviour in callus culture of Zea mays L. Cytologia 51:37–41
Murashige T, Skoog F (1962) A revised medium for rapid growth and biossays with tobbacco tissue cultures. Physiol Plant 15:437–497
Murata M (1991) Cytogenetic changes during seed storage. In: Gupta PK, Tsuchiya T (eds) Chromosome engineering in plants: genetics, breeding, evolution, part A vol. 2A. Elsevier, Amsterdam Oxford New York Tokyo, pp 211–228
Murata M, Orton TJ (1984) Chromosome fusions in cultured cells of celery. Can J Genet Cytol 26:395–400
Murata M, Tsuchiya T, Roos EE (1984) Chromosome damage induced by artificial seed aging in barley. 3. Behavior of chromosomal aberrations during plant growth. Theor Appl Genet 67:161–170
Peschke VM, Phillips RL (1991) Activation of the maize transposable element Suppressor-mutator (Spm) in tissue culture. Theor Appl Genet 81:90–97
Peschke VM, Phillips RL (1992) Genetic implications of somaclonal variation in plants. Adv Genet 30:41–75
Peschke VM, Phillips RL, Gengenbach BG (1987) Discovery of transposable element activity among progeny of tissue culture-derived maize plants. Science 238:804–807
Peschke VM, Phillips RL, Gengenbach BG (1991) Genetic and molecular analysis of tissue culture-derived Ac elements. Theor Appl Genet 82:121–129
Peterson CL (1994) The SMC family: novel motor proteins for chromosome condensation? Cell 79:389–392
Phillips RL, Somers DA, Hibberd KA (1988) Cell/tissue culture and in vitro manipulation. In: Sprague GF, Dudley JW (eds) Corn and corn improvement 3rd edn. ASA/CSSA/SSSA, Madison, pp 345–387
Phillips RL, Kaeppler SM, Peschke VM (1990) Do we understand somaclonal variation? In: Nijkamp HJJ, Van der Plas LHW, Van Aartrijk J (eds) Progress in plant cellular and molecular biology. Proc Intl Cong Plant Tissue Cell Cult vol. 7. Kluwer Academic, Dordrecht Boston London, pp 131–141
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci USA 91:5222–5226
Priestley DA (1986) Seed aging — implications for seed storage and persistence in the soil. Comstock Publ Assoc, Cornell Univ Press, Ithaca, N. Y.
Pryor A, Faulkner K, Rhoades MM, Peacock WJ (1980) Asynchronous replication of heterochromatin in maize. Proc Natl Acad Sci USA 77:6705–6709
Rhodes CA, Phillips RL, Green CE (1986) Cytogenetic stability of aneuploid maize tissue cultures. Can J Genet Cytol 28:374–384
Sacristán MD (1971) Karyotypic changes in callus cultures from haploid and diploid plants of Crepis capillaris (L) Wallr. Chromosoma 33:273–283
Singh RJ (1993) Plant cytogenetics. CRC Press, Boca Raton Ann Arbor London Tokyo
Uemura T, Ohkura H, Adachi Y, Morino K, Shiozaki K, Yanagida M (1987) DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell 50:917–925
Vasil IK (1986) Developing cell and tissue culture systems for the improvement of cereal and grass crops. J Plant Physiol 128:193–218
Watt PM, Hickson ID (1994) Structure and function of type II DNA topoisomerases. Biochem J 303:681–695
Author information
Authors and Affiliations
Additional information
Communicated by F. Salamini
Rights and permissions
About this article
Cite this article
Fluminhan, A., Kameya, T. Behaviour of chromosomes in anaphase cells in embryogenic callus cultures of maize (Zea mays L.). Theoret. Appl. Genetics 92, 982–990 (1996). https://doi.org/10.1007/BF00224038
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00224038