Abstract
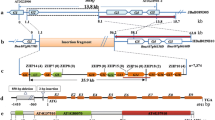
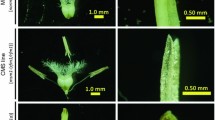
We previously mapped one male-sterile gene (Bnms3) from an extensively used recessive genic male sterility line (9012AB) in Brassica napus to a 0.14-cM genomic region. In this study, two highly homologous BAC contigs possibly containing the candidate BnMs3 gene were identified using a map-based cloning strategy. A BnMs3-linked SCAR marker (DM1) capable of differentiating the subgenomes between B. rapa and the B. oleracea aided mapping of BnMs3 on the contig derived from the B. napus chromosome C9. One representative BAC clone was sequenced from each of the two contigs and resulted in a larger number of markers according to the sequence difference between the two clones. To isolate BnMs3, these markers were then analyzed in another two BC1 populations with different genetic backgrounds. This assay allowed for a delimitation of the mutated functional region of BnMs3 to a 9.3-kb DNA fragment. Gene prediction suggested that one complete open reading frame (ORF, ORF2) and partial CDS fragments of ORF1 and ORF3 reside in this fragment. Sequence comparison and genetic transformation eventually indicated that ORF1 (designated as BnaC9.Tic40), an analogue of the Arabidopsis gene AT5G16620 which encodes a translocon of the inner envelope of chloroplasts 40 (Tic40), is the only candidate gene of BnMs3. Furthermore, two distinct mutation types in ORF1 both causing the male-sterile phenotype were individually revealed from 9012A and the temporary maintainer line T45. The molecular mechanism of this male sterility as well as the application of BnMs3-associated functional and cosegregated markers in true breeding programs was also discussed.


Similar content being viewed by others
References
Brown GG, Formanuvá N, Jin H, Wargachuk R, Dendy C, Patil P, Laforest M, Zhang JF, Cheung WY, Landry BS (2003) The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J 35:262–272
Cardoza V, Stewart CN (2003) Increased Agrobacterium-mediated transformation and rooting efficiencies in canola (Brassica napus L.) from hypocotyl segment explants. Plant Cell Rep 21:599–604
Chen FX, Hu BC, Li QS (1993) Discovery and study of genic male sterility (GMS) material 9012A in Brassica napus L (in Chinese). Acta Agric Univ Pekinensis 19(Suppl):57–61
Chen FX, Hu BC, Li C, Li QS, Chen WS, Zhang ML (1998) Genetic studies on GMS in Brassica napus L: I. Inheritance of recessive GMS line 9012A. Acta Agron Sin 24:431–438
Desloire S, Gherbi H, Laloui W, Marhadour S, Clouet V, Cattolico L, Falentin C, Giancola S, Renard M, Budar F, Small I, Caboche M, Delourme R, Bendahmane A (2003) Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family. EMBO Rep 4:588–594
Dong FM, Hong DF, Liu PW, Xie YZ, He QB, Yang GS (2010) A novel genetic model for recessive genic male sterility line 9012AB in rapeseed (Brassica napus L.). J Huazhong Agric Univ 29:262–267
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Dun XL, Zhou ZF, Xia SQ, Wen J, Yi B, Shen JX, Ma CZ, Tu JX, Fu TD (2011) BnaC.Tic40, a plastid inner membrane translocon originating from Brassica oleracea, is essential for tapetal function and microspore development in Brassica napus. Plant J 68:532–545
Formanová N, Li XQ, Alison MRF, DePauw M, Keller WA, Landry B, Brown GG (2006) Towards positional cloning in Brassica napus: generation and analysis of doubled haploid B. rapa possessing the B. napus pol CMS and Rfp nuclear restorer gene. Plant Mol Biol 61:269–281
Frauen M, Noack J, Girke A, Paulmann W (2007) Ten years experience of development and cultivation of winter oilseed rape hybrids in Europe based on the MSL system Poc 12th Int Rapeseed Congress, Wuhan, China
He JP, Ke LP, Hong DF, Xie YZ, Wang GC, Liu PW, Yang GS (2008) Fine mapping of a recessive genic male sterility gene (Bnms3) in rapeseed (Brassica napus) with AFLP- and Arabidopsis-derived PCR markers. Theor Appl Genet 117:11–18
Howell EC, Kearsey MJ, Jones GH, King GJ, Armstrong SJ (2008) A and C genome distinction and chromosome identification in Brassica napus by sequential fluorescence in situ hybridization and genomic in situ hybridization. Genetics 180:1849–1857
Huang Z, Chen YF, Yi B, Xiao L, Ma CZ, Tu JX, Fu TD (2007) Fine mapping of the recessive genic male sterility gene (Bnms3) in Brassica napus L. Theor Appl Genet 115:113–118
Ke LP, Sun YQ, Hong DF, Liu PW, Yang GS (2005) Identification of AFLP markers linked to one recessive genic male sterility gene in oilseed rape, Brassica napus L. Plant Breed 124:367–370
Le Cunff L, Garsmeur O, Marie Raboin L et al (2008) Diploid/polyploid syntenic shuttle mapping and haplotype-specific chromosome walking toward a rust resistance gene (Bru1) in highly polyploid sugarcane (2n ~ 12x ~ 115). Genetics 180:649–660
Li HM, Chiu CC (2010) Protein transport into chloroplasts. Annu Rev Plant Biol 61:157–180
Long Y, Shi J, Qiu D, Li R, Zhang C, Wang J, Hou J, Zhao J, Shi L, Beom-Seok Park, Choi SR, Lim YP, Meng J (2007) Flowering time quantitative trait loci analysis of oilseed Brassica in multiple environments and genomewide alignment with Arabidopsis. Genetics 177:2433–2444
Lu S, Eck JV, Zhou XJ et al (2006) The cauliflower Or gene encodes a DnaJ Cysteine-rich domain-containing protein that mediates high levels of β-carotene accumulation. Plant Cell 18:3594–3605
Lysak MA, Koch MA, Beaulieu JM, Meister A, Leitch IJ (2009) The dynamic ups and downs of genome size evolution in Brassicaceae. Mol Biol Evol 26:85–98
Mariani C, Gossele V, De Beuckeleer M, De Block M, Goldberg RB, De Greef W, Leemans J (1992) A chimaeric ribonuclease-inhibitor gene restores fertility to male sterile plants. Nature 357:384–387
Muangprom A, Osborn TC (2004) Characterization of a dwarf gene in Brassica rapa, including the identification of a candidate gene. Theor Appl Genet 108:1378–1384
Ogura H (1968) Studies on the male sterility in Japanese radish, with special references to the utilization of this sterility towards practical raising of hybrid seed. Mem Fac Agric Kagoshima Univ 6:39–78
Osborn TC, Butrulle DV, Sharpe AG, Pickering KJ, Parkin IAP, Parker JS, Lydiate DJ (2003) Detection and effects of a homeologous reciprocal transposition in Brassica napus. Genetics 165:1569–1577
Ostergaard L, King GJ (2008) Standardized gene nomenclature for the Brassica genus. Plant Methods 4:10
Panjabi P, Jagannath A, Bisht NC, Padmaja KL, Sharma S, Gupta V, Pradhan AK, Pental D (2008) Comparative mapping of Brassica juncea and Arabidopsis thaliana using Intron Polymorphism (IP) markers: homoeologous relationships, diversification and evolution of the A, B and C Brassica genomes. BMC Gemomics 9:113
Parkin IAP, Gulden SM, Sharpe AG, Lukens L, Trick M, Osborn TC, Lydiate DJ (2005) Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana. Genetics 171:765–781
Rana DT, van den Boogaart T, O’Neill CM, Hynes L, Bent E, Macpherson L, Park JY, Lim YP, Bancroft I (2004) Conservation of the microstructure of genome segments in Brassica napus and its diploid relatives. Plant J 40:725–733
Schranz ME, Lysak MA, Mitchell-Olds T (2006) The ABC’s of comparative genomics in the Brassicaceae: building blocks of crucifer genomes. Trends Plant Sci 11(11):535–542
Stiewe G, Pleines S, Coque M, Gielen J (2010) New hybrid system for Brassica napus. US patent application 20100222605
Su PH, Li HM (2010) Stromal Hsp70 is important for protein translocation into Pea and Arabidopsis chloroplasts. Plant cell 22:1516–1531
Town CD, Cheung F, Maiti R, Crabtree J, Haas BJ, Wortman JR, Hine EE, Althoff R, Arbogast TS, Tallon LJ, Vigouroux M, Trick M, Bancroftb I (2006) Comparative genomics of Brassica oleracea and Arabidopsis thaliana reveal gene loss, fragmentation, and dispersal after polyploidy. Plant Cell 18:1348–1359
Viana AAB, Li M, Schnell DJ (2010) Determinants for stop-transfer and post-import pathways for protein targeting to the chloroplast inner envelope membrane. J Biol Chem 285:12948–12960
Wan LL, Xia XY, Hong DF, Li J, Yang GS (2010) Abnormal vacuolization of the tapetum during the tetrad stage is associated with male sterility in the recessive genic male sterile Brassica napus L. line 9012A. J. Plant Biol 53:121–133
Wang GC, He JP, Hong DF, Xie YZ, Xu ZH, Liu PW, Yang GS (2007) Development of AFLP and SCAR markers linked to a recessive genic male sterile gene (ms3) in rapeseed for marker-assisted selection. Korean J Genetics 29:481–487
Wang J, Long Y, Wu BD, Liu J, Jiang CC, Shi L, Zhao JW, King GJ, Meng JL (2009) The evolution of Brassica napus FLOWERING LOCUST paralogues in the context of inverted chromosomal duplication blocks. BMC Evol Biol 9:27
Wang J, Lydiate DJ, Parkin IAP, Falentin C, Régine Delourme, Carion PWC, King GJ (2011) Integration of linkage maps for the amphidiploids Brassica napus and comparative mapping with Arabidopsis and Brassica rapa. BMC Genomics 12:101
Williams ME, Lecmans J, Michiels F (1997) Male sterility through recombinant DNA technology. In: Shivanna KR, Sawhney VK (eds) Pollen biotechnology for crop production and improvement. Cambridge Univ Press, London, pp 237–257
Xiao L, Yi B, Chen YF, Huang Z, Chen W, Ma CZ, Tu JX, Fu TD (2008) Molecular markers linked to Bn;rf: a recessive epistatic inhibitor gene of recessive genic male sterility in Brassica napus L. Euphytica 164:377–384
Xie YZ, Hong DF, Xu ZH, Liu PW, Yang GS (2008) Identification of AFLP markers linked to the epistatic suppressor gene of a recessive genic male sterility in rapeseed and conversion to SCAR markers. Plant Breed 127:145–149
Xu ZH, Xie YZ, Hong DF, Liu PW, Yang GS (2009) Fine mapping of the epistatic suppressor gene (esp) of a recessive genic male sterility in rapeseed (Brassica napus L.). Genome 52:755–760
Yang GS, Fu TD (1987) Environment effects on the cytoplasmic male sterility of rapeseed (Brassica napus and Brassica campestris). Oil Crops of China 3:15–19
Yang GS, Qu B, Fu TD (1999) Cytological study of microsporogenesis in three recessive genic male sterile lines of Brassica napus L. J Huazhong Agric Univ 18:520–530
Yi B, Zeng FQ, Lei SL, Chen YN, Yao XQ, Zhu Y, Wen J, Shen JX, Ma CZ, Tu JX, Fu TD (2010) Two duplicate CYP704B1-homologous genes BnMs1 and BnMs2 are required for pollen exine formation and tapetal development in Brassica napus. Plant J 63:925–938
Zu F, Xia SQ, Dun XL, Zhou ZF, Zeng FQ, Yi B, Wen J, Ma CZ, Shen JX, Tu JX, Fu TD (2010) Analysis of genetic model for a recessive genic male sterile line 7–7365AB in Brassica napus L. based on molecular markers. Scientia Agricultura Sinica 43:3067–3075
Acknowledgments
The authors wish to thank Prof. Jinling Meng for kindly providing the BAC clones of the JBnB BAC library and genomic DNA of Tapidor as well as eight B. oleracea lines. We also thank Prof. Kede Liu for providing the genomic DNA of 50 B. rapa lines used in this research and Dr. Zhixiong Fan for providing the genomic DNA of the temporary maintainer line 9012B-6DH. This research was supported by Natural Science Foundation of China (30670123, 31170166) and National High-tech R&D Program of China (2011AA10A104).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Becker.
J. Li and D. Hong contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Hong, D., He, J. et al. Map-based cloning of a recessive genic male sterility locus in Brassica napus L. and development of its functional marker. Theor Appl Genet 125, 223–234 (2012). https://doi.org/10.1007/s00122-012-1827-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-012-1827-5