Abstract
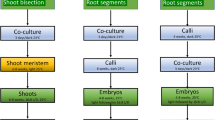
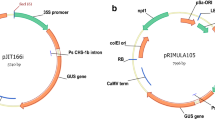
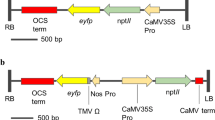
Transgenic plants frequently exhibit altered phenotypes, unrelated to transgene expression, which are attributed to tissue culture-induced variation and/or insertional mutagenesis. Distinguishing between these possibilities has been difficult in clonal crops such as potato, due to their highly heterozygous background and the resulting inherent phenotypic variability associated with segregation. This study reports the use of transgene integration as a molecular marker to trace the clonal origin of single cells in tissue culture. Following transformation, multiple shoots have been regenerated from cell colonies of potato (Solanum tuberosum L.) and Southern analysis used to confirm their derivation from a single transformed cell. Analysis of phenotypic variation in field trials has demonstrated marked differences between these multiple regeneration events, the origin of which must have occurred after T-DNA insertion, and consequently during the tissue culture phase. This result unequivocally demonstrates that somaclonal variation occurs during tissue culture and independent of transgene insertion. Furthermore, the first shoots recovered do not necessarily exhibit less somaclonal variation, since later regeneration events can give rise to plants that are more phenotypically normal. Therefore, when developing transgenic lines for genetic improvement of clonal crops, multiple shoots should be regenerated and evaluated from each transformation event to facilitate the recovery of phenotypically normal transgenic lines.


Similar content being viewed by others
References
Barrell PJ, Conner AJ (2009) Expression of a chimeric magainin gene in potato confers improved resistance to the phytopathogen Erwinia carotovora. Open Plant Sci J 3:14–21
Barrell PJ, Shang YJ, Cooper PA, Conner AJ (2002) Alternative selectable markers for potato transformation using minimal T-DNA vectors. Plant Cell Tiss Organ Cult 70:61–68
Belknap WR, Corsini D, Pavek JJ, Snyder GW, Rockhold R and Vayda, ME (1994) Field performance of transgenic Russet Burbank and Lemhi Russet potatoes. Am Potato J 71:285–296
Conner AJ (2007) Field-testing of transgenic potatoes. In: Vreugdenhil D (ed) Potato biology, biotechnology: advances, perspectives. Elsevier, Amsterdam, pp 687–703
Conner AJ, Christey MC (1994) Plant breeding and seed marketing options for the introduction of transgenic insect-resistant crops. Biocontrol Sci Tech 4:463–473
Conner AJ, Williams MK, Abernethy DJ, Fletcher PJ, Genet RA (1994) Field performance of transgenic potatoes. N Z J Crop Hort Sci 22:361–371
Dale PJ, McPartlan HC (1992) Field performance of transgenic potato plants compared with controls regenerated from tuber discs and shoot cuttings. Theor Appl Genet 84:585–591
Davidson MM, Jacobs JME, Reader JK, Butler RC, Frater CM, Markwick NP, Wratten SD, Conner AJ (2002a) Development and evaluation of potatoes transgenic for a cry1Ac9 gene conferring resistance to potato tuber moth. J Am Soc Hort Sci 127:590–596
Davidson MM, Takla MFG, Reader JK, Butler RC, Wratten SD, Conner AJ (2002b) Evaluation of field grown potato lines transgenic for a cry1Ac9 gene conferring resistance to potato tuber moth. N Z Plant Prot 55:405–410
Deroles SC, Gardner RC (1988) Expression and inheritance of kanamycin resistance in a large number of transgenic petunias generated by Agrobacterium-mediated transformation. Plant Mol Biol 11:355–364
Duncan RR (1997) Tissue culture-induced variation and crop improvement. Adv Agron 58:201–240
Gleave AP, Mitra DS, Markwick NP, Morris BAM, Beuning LL (1998) Enhanced expression of the Bacillus thuringiensis cry9Aa2 gene in transgenic plants by nucleotide sequence modification confers resistance to potato tuber moth. Mol Breed 4:459–472
Heeres P, Schippers-Rozenboom M, Jacobsen E, Visser RGF (2002) Transformation of a large number of potato varieties: genotype-dependent variation in efficiency and somaclonal variation. Euphytica 124:13–22
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Howard HW (1970) Genetics of the potato-Solanum tuberosum. Logos Press, London
Jongedijk E, de Schutter AAJM, Stolte T, van den Elzen PJM, Cornelissen BJC (1992) Increased resistance to potato virus X and preservation of cultivar properties in transgenic potatoes under field conditions. Bio/Technology 10:422–429
Karp A (1991) On the current understanding of somaclonal variation. Oxford Surv Plant Mol Cell Biol 7:1–58
Larkin PJ, Scowcroft WR (1981) Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Morgan ER, Burge GK, Seelye JF, Grant JE, Hopping ME (1995) Interspecific hybridisation between Limonium perigrinum Bergius and Limonium purpuratum L. Euphytica 83:215–224
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarium 15:473–497
Phillips RL, Kaeppler SM and Olhoft P (1994) Genetic instability of plant tissue cultures: Breakdown of normal controls. In: Proceedings of the National Academy of Sciences (USA) 91:5222–5226
Potter R, Jones MGK (1991) An assessment of genetic stability of potato in vitro by molecular and phenotypic analysis. Plant Sci 76:239–248
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Secor GA, Shepard JF (1981) Variability of protoplast-derived potato clones. Crop Sci 21:102–105
Shepard JF, Bindey D, Shahin E (1980) Potato protoplasts in crop improvement. Science 208:17–24
Simmonds NW (1979) Principles of plant breeding. Longman, London
Sokal RR, Rolf FJ (1969) Biometry: the principles and practices of statistics in biological research. Freeman, San Francisco
Thieme R, Griess H (2005) Somaclonal variation in tuber traits of potato. Potato Research 48:153–165
Van Engelen FA, Molthoff JW, Conner AJ, Nap JP, Pereira A, Stiekema WJ (1995) pBINPLUS: an improved plant transformation vector based on pBIN19. Transgenic Res 4:288–290
Veilleux RE, Johnson AAT (1998) Somaclonal variation: molecular analysis, transformation interaction, and utilization. Plant Breed Rev 16:229–268
Acknowledgments
We thank Jill Reader and Ruth Butler for assistance with the field trials and statistical analysis respectively; Tonya Frew for help with probing Southern blots; and Sathiyamoorthy Meiyalaghan and Jeanne Jacobs for valuable discussions and comments on earlier drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gebhardt.
Rights and permissions
About this article
Cite this article
Barrell, P.J., Conner, A.J. Facilitating the recovery of phenotypically normal transgenic lines in clonal crops: a new strategy illustrated in potato. Theor Appl Genet 122, 1171–1177 (2011). https://doi.org/10.1007/s00122-010-1521-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-010-1521-4