Abstract
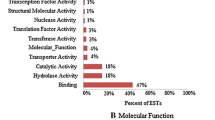
To clarify the mechanisms of stress tolerance in rice and to search for rice genes associated with these mechanisms, we analyzed genes induced by a high salinity treatment using the PCR-subtractive hybridization method (PCR-subtraction). Seedlings of the salt-tolerant rice cultivar Dee-geo-woo-gen (DGWG) were either treated with 250 mM NaCl for 5 h or left untreated, and PCR-subtraction was then performed using the untreated (control) plants as a driver and the NaCl-treated plants as a tester. We obtained 384 clones of tester-specific cDNAs as salt-inducible candidates. Northern analysis performed with the cDNA fragments showed that 65 clones had been induced by the NaCl treatment. Sequence analysis and database searching indicated that these clones have homology to proteins functional for detoxification, stress response, and signal transduction in plants. Of these clones, 22% coded for unknown proteins and 12% gave no hits. We selected eight clones from each functional category and analyzed their expression pattern in DGWG. For temporal analysis, seedlings were treated with H2O or 250 mM NaCl for 0, 0.5, 1, 2, 5, 10 or 24 h. Different patterns of transcript regulation were found. For the analysis of expression in response to various types of stress and abscisic acid (ABA) treatments, seedlings were treated for 5 h or 10 h with H2O, dehydration, cold (4°C), heat (40°C), mannitol, ABA, or wounding. All clones were strongly up-regulated by osmotic stress (dehydration and mannitol) and the ABA treatment.


Similar content being viewed by others
References
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Chen CN, Porubleva L, Shearer G, Svrakic M, Holden LG, Dover JL, Johnston M, Chitnis PR, Kohl DH (2003) Associating protein activities with their genes: rapid identification of a gene encoding a methylglyoxal reductase in the yeast Saccharomyces cerevisiae. Yeast 20:545–554
Chung WS, Lee SH, Kim JC, Heo WD, Kim MC, Park CY, Park HC, Lim CO, Kim WB, Harper JF, Cho MJ (2000) Identification of a calmodulin-regulated soybean Ca(2+)-ATPase (SCA1) that is located in the plasma membrane. Plant Cell 12:1393–1407
Deleu C, Coustaut M, Niogert MF and Larher F (1999) Three new osmotic stress-regulated cDNAs identified by differential display polymerase chain reaction in rapeseed leaf discs. Plant Cell Environ 22:979–988
Galili G, Tang G, Zhu X, Gakiere B (2001) Lysine catabolism: a stress and development super-regulated metabolic pathway. Curr Opin Plant Biol 4:261–266
Garcia AB, Engler J, Iyer S, Gerats T, Van Montagu M, Caplan AB (1997) Effects of osmoprotectants upon NaCl stress in rice. Plant Physiol 115:159–169
Hanson AD, Rathinasabapathi B, Rivoal J, Burnet M, Dillon MO, Gage DA (1994) Osmoprotective compounds in the Plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance. Proc Natl Acad Sci USA 91:306–310
Holmstrom KO, Somersalo S, Mandal A, Palva TE, Welin B (2000) Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot 51:177–185
Igarashi Y, Yoshiba Y (2001) Improving the salt tolerance of proline-accumulated rice by suppressing Na+ absorption. Rice Genet Newsl 17:69–72
Igarashi Y, Yoshiba Y, Sanada Y, Yamaguchi-Shinozaki K, Wada K, Shinozaki K (1997) Characterization of the gene for delta1-pyrroline-5-carboxylate synthetase and correlation between the expression of the gene and salt tolerance inOryza sativa L. Plant Mol Biol 33:857–65
Ishitani M, Xiong L, Stevenson B, Zhu JK (1997) Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell 9:1935–1949
Jeong SW, Choi SM, Lee DS, Ahn SN, Hur Y, Chow WS, Park YI (2002) Differential susceptibility of photosynthesis to light-chilling stress in rice (Oryza sativa L.) depends on the capacity for photochemical dissipation of light. Mol Cell 13:419–428
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17:287–291
Kawasaki S, Borchert C, Deyholos M, Wang H, Brazille S, Kawai K, Galbraith D, Bohnert HJ. (2001) Gene expression profiles during the initial phase of salt stress in rice. Plant Cell 13:889–905
Kishor P, Hong Z, Miao GH, Hu C, Verma D (1995) Overexpression of [delta]-pyrroline-5-carboxylate synthetase Increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394
Lee JH, Chae HS, Lee JH, Hwang B, Hahn KW, Kang BG, Kim WT (1997) Structure and expression of two cDNAs encoding S-adenosyl-L-methionine synthetase of rice (Oryza sativa L.). Biochim Biophys Acta 1354:13–18
Maiale S, Sanchez DH, Guirado A, Vidal A, Ruiz OA (2004) Spermine accumulation under salt stress. J Plant Physiol 161:35–42
Maiti MK, Krishnasamy S, Owen HA, Makaroff CA (1997) Molecular characterization of glyoxalase II from Arabidopsis thaliana. Plant Mol Biol 35:471–481
Mittova V, Tal M, Volokita M, Guy M (2003) Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 26:845–856
Moons A, Bauw G, Prinsen E, Van Montagu M, Van der Straeten D (1995) Molecular and physiological responses to abscisic acid and salts in roots of salt-sensitive and salt-tolerant Indica rice varieties. Plant Physiol 107:177–186
Nakashima K, Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K (1997) A nuclear gene, erd1, encoding a chloroplast-targeted Clp protease regulatory subunit homolog is not only induced by water stress but also developmentally up-regulated during senescence in Arabidopsis thaliana. Plant J 12:851–861
Nakazono M, Tsuji H, Li Y, Saisho D, Arimura S, Tsutsumi N, Hirai A (2000) Expression of a gene encoding mitochondrial aldehyde dehydrogenase in rice increases under submerged conditions. Plant Physiol 124:587–598
Nanjo T, Kobayashi M, Yoshiba Y, Sanada Y, Wada K, Tsukaya H, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (1999) Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J 18:185–193
Noiraud N, Delrot S, Lemoine R (2000) The sucrose transporter of celery. Identification and expression during salt stress. Plant Physiol 122:1447–1455
Oztur ZN, Talame V, Deyholos M, Michalowski CB, Galbraith DW, Gozukirmizi N, Tuberosa R, Bohnert HJ (2002) Monitoring large-scale changes in transcript abundance in drought- and salt-stressed barley. Plant Mol Biol 48:551–573
Pillai MA, Akiyama T (2004) Differential expression of an S-adenosyl-L-methionine decarboxylase gene involved in polyamine biosynthesis under low temperature stress in japonica and indica rice genotypes. Mol Genet Genomics 271:141–149
Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol 133:1755–1767
Reyes BG de los, Morsy M, Gibbons J, Varma TS, Antoine W, McGrath JM, Halgren R, Redus M (2003) A snapshot of the low temperature stress transcriptome of developing rice seedlings (Oryza sativa L.) via ESTs from subtracted cDNA library. Theor Appl Genet 107:1071–1082
Sahi C, Agarwal M, Reddy MK, Sopory SK, Grover A (2003) Isolation and expression analysis of salt stress-associated ESTs from contrasting rice cultivars using a PCR-based subtraction method. Theor Appl Genet 106:620–628
Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Taji T, Yamaguchi-Shinozaki K, Carninci P, Kawai J, Hayashizaki Y, Shinozaki K (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high salinity stresses using a full-length cDNA microarray. Plant J 31:279–292
Shen Q, Zhang P, Ho TH (1996) Modular nature of abscisic acid (ABA) response complexes: composite promoter units that are necessary and sufficient for ABA induction of gene expression in barley. Plant Cell 8:1107–1119
Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3:217–223
Sunkar R, Bartels D, Kirch HH (2003) Overexpression of a stress-inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J 35:452–464
Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2002) Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J 29:417–426
Ueda A, Shi W, Nakamura T, Takabe T (2002) Analysis of salt-inducible genes in barley roots by differential display. J Plant Res 115:119–130
Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97:11632–11537
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Wise MJ (2003) LEAping to conclusions: A computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4:52
Wise MJ, Tunnacliffe A (2004) POPP the question: what do LEA proteins do? Trends Plant Sci 9:13–17
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14[Suppl]:S165–183
Xu D, Duan X, Wang B, Hong B, Ho T, Wu R (1996) Expression of a Late Embryogenesis Abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol 110:249–257
Yamakawa S, Matsubayashi Y, Sakagami Y, Kamada H, Satoh S (1999) Promotive effects of the peptidyl plant growth factor, phytosulfokine-alpha, on the growth and chlorophyll content of Arabidopsis seedlings under high night-time temperature conditions. Biosci Biotechnol Biochem 63:2240–2243
Yang H, Matsubayashi Y, Nakamura K, Sakagami Y (1999) Oryza sativa PSK gene encodes a precursor of phytosulfokine-alpha, a sulfated peptide growth factor found in plants. Proc Natl Acad Sci USA 96:13560–13565
Yoshiba Y, Kiyosue T, Katagiri T, Ueda H, Mizoguchi T, Yamaguchi-Shinozaki K, Wada K, Harada Y, Shinozaki K (1995) Correlation between the induction of a gene for delta 1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J 7:751–760
Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (1997) Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol 38:1095–1102
Acknowledgements
This work was supported by a project grant of the Bio-oriented Technology Research Advancement Institution (BRAIN).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Guiderdoni
Rights and permissions
About this article
Cite this article
Shiozaki, N., Yamada, M. & Yoshiba, Y. Analysis of salt-stress-inducible ESTs isolated by PCR-subtraction in salt-tolerant rice. Theor Appl Genet 110, 1177–1186 (2005). https://doi.org/10.1007/s00122-005-1931-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-005-1931-x